Abstract
Populations in the global south are disproportionately exposed to the stressors of development, disaster and armed conflict, all of which heighten cardiovascular disease (CVD) risk. We consider how war-related stressors exert a lasting influence upon population health, in particular the cardiovascular health of war survivors now entering older adulthood. Data come from the 2018 Vietnam Health and Aging Study conducted among 2,447 northern Vietnamese adults age 60 and older. We conduct survey-adjusted logistic regression analyses to examine the associations among respondents’ wartime exposure to combat and physical threat, malevolent environment conditions, and four CVD conditions (hypertension, dyslipidemia, heart disease, and stroke). We examine posttraumatic stress disorder (PTSD) as it mediates the association between wartime stress exposures and late life CVD, and gender as it moderates the relationship between wartime stressors and CVD. We find that exposure to wartime combat and violence, as well as malevolent living conditions, exhibit significant, positive associations with cardiovascular conditions. These associations are mediated by the severity of recent PTSD symptoms. For certain CVD conditions, particularly hypertension, the associations between wartime stressors and late life cardiovascular conditions diverge across gender with women experiencing a greater penalty for their exposure to war-related stressors than their male counterparts. We conclude that the stressors of war and resultant PTSD, widespread in this cohort of Vietnamese older adults who endured myriad forms of war exposure during their young adulthood, exhibit modest, yet significant associations with late-life cardiovascular conditions. Women, especially those exposed to wartime violence and combat, bear this CVD burden alongside men.
Keywords: Vietnam, Cardiovascular Disease, Vietnam War, Posttraumatic Stress Disorder, Gender, Hypertension, Older Adulthood, Health Determinants
Introduction:
Early Life War Exposure and Health in Late Life
The global disease burden of armed conflict is significant and weighs most heavily upon low and middle-income countries (LMICs) where war-damaged infrastructure often layers upon fragile economies and institutions (Jawad 2019; Murray et al. 2002; Roth et al. 2018). The toll of armed conflict increasingly affects noncombatants and burdens shelter, medical care and human security, resulting in long-term population-wide excess mortality and morbidity (Poole 2012; Johnson 2017; Wise 2017). Yet, in assessing the morbidity and mortality resulting from war, researchers have often focused narrowly upon deaths resulting directly from the violence of conflict, taking place during or immediately following wars (Roth et al. 2018). Others focus upon health outcomes, especially infectious disease and mental health, in refugee situations proximate to war (e.g., Spiegel et al. 2010). Globally, a wide array of countries, Vietnam being illustrative, experienced protracted wars which exposed widespread segments of their populations to diverse, often extreme, forms of violence and trauma. Guided by past research conducted within veteran populations, we propose that understanding of health disparities and chronic disease risks in LMICs with rapidly aging populations can be greatly enhanced through a life course lens on early life exposure to the stressors of war. We utilize a novel data source to examine the enduring effects of war upon cardiovascular health in a cohort of older adults in northern Vietnam who, deeply and diversely exposed to wartime violence, are now entering older adulthood and experiencing heightened risk of chronic disease.
Assessments of armed conflict’s consequences for morbidity and mortality depend upon the frameworks adopted by analysts, the populations chosen for study, and the timeframes within which conflict is ascertained as a cause of death and disease. Two studies illustrate how methodological decisions influence our understanding of war’s broader health impacts. In an early study of wartime determinants of CVD, Sibai and colleagues (1989) observed that, beyond the stressors of combat, daily hassles faced by civilians in Lebanon, in particular the crossing of “green-lines” between warring sides in its civil war, constituted a stressor associated with elevated coronary artery disease. Recently, an investigation of infant and child mortality across armed conflict events in Africa from 1995–2015 demonstrated that the infant death toll of conflict is remarkably higher when the radius of “exposed” communities is drawn more broadly and when children are followed not just one, but ten years, post-conflict (Wagner et al. 2018). These studies suggest that we risk underestimating the consequences of war if our analyses are limited to the immediate post-conflict period and ignore the wide spectrum of exposures that vary geographically, and across segments of population. As frameworks and methodologies zoom out to assess indirect deaths due to conflict, especially those among noncombatants, the scale often far exceeds that of the immediate, direct impact (Hagopian et al. 2013; Wise 2017).
Myriad studies adopting a life course perspective illustrate the lifelong influence of military service upon health and mortality risks (Settersten 2006; Chatterjee et al. 2009; Elder et al. 2009). These studies suggest that morbidities and mortality in older adulthood bear the stamp of war exposures experienced by veterans in adolescence and early adulthood (e.g., Beristianos et al. 2016; Schnurr and Spiro 1999; Taylor et al. 2016). The “long arm” of war in early life is also evident in studies of morbidities among individuals affected by severe wartime famine and consequent malnutrition (e.g., Sparen et al. 2004; Stein et al. 1975).
We can meaningfully extend the conceptualization of war and military experiences as powerful “hidden variables” to analyze health in late adulthood across a wide set of post-conflict settings (Settersten 2006). Investigations of the more distal or ‘remote’ exposures to war as they influence war survivors’ health in late life are needed in order to more fully ascertain the enduring consequences of war for population health (Wise 2017). Older adults who have survived wars live throughout the world and their numbers are increasing. Their illnesses and injuries are potentially as challenging to societies as war deaths and as crucial to understand, given the ongoing treatment and adaptation they demand.
War, Posttraumatic Stress & CVD
Cardiovascular disease (CVD) is a critical noncommunicable disease outcome for understanding how war “gets under skin” to influence aging and disease processes among survivors. Rising CVD is of utmost concern in LMICs, where the disease accounts for a rising share of deaths and remains widely underdiagnosed and untreated, resulting in elevated health care costs and mortality rates (Anand et al. 2020). While wealthy nations of the global north have witnessed moderate declines in the burden of CVD, many LMICs are seeing CVD deaths rise dramatically in association with the demographic and social changes that accompany development, such as population aging, sedentary lifestyles, tobacco use, and dietary changes that heighten obesity and metabolic syndrome (Roth et al. 2015; Finegold et al. 2013). As in other LMICs, high levels of CVD are frequently undiagnosed and untreated in Vietnam. As Van Minh and colleagues (2006) observed in the study of one peri-urban district of northern Vietnam, a full 83% of hypertensives were unaware of their high blood pressure. Understanding which LMIC populations and subpopulations are at risk, and the unique factors within LMICS that raise CVD risk, will be important to mount viable and effective CVD screening and prevention efforts.
Wartime stress exposures are arguably a critical element of CVD risk profiles in post-conflict LMICs where stressful war exposures extend across wide segments of older adults. A recent scoping review (Jawad et al. 2019) suggests that rising CVD burden in a host of LMICs is intertwined with the experience of armed conflict. While increasing, Jawad and colleagues (2019:1393) note that the armed conflict-CVD relationship has received “a paucity of attention” in academic literature, with the few existing studies often lacking sufficiently rigorous methods, and tending to “homogenize” armed conflict “as a simplistic exposure variable.” Relatively few studies attend to specific war exposures and mechanisms, such as the role of stress response as it relates to certain behavioral and physiological CVD risks.
Past research among American veterans of the Vietnam War and other veteran populations have observed an association between war-related stress exposures, especially experiences of combat deployment, and CVD (Vaccarino et al. 2013; Kang et al. 2006). Many such studies further suggest that PTSD, or the psychological response to trauma exposure, acts as a chief mediator of the relationship between wartime service, trauma exposure, and CVD (Boscarino 2006; Britvic et al. 2015; Kang et al. 2006; Moazen-Zadeh et al. 2016). These studies point to both physiological and psychological pathologies that often occur alongside PTSD and that heighten risk for CVD (Beristianos et al. 2016; Kibler et al. 2009; O’Toole and Catts 2008; Schnurr and Spiro 1999). For instance, widely documented biological abnormalities associated with PTSD, such as heightened blood pressure, cardiovascular reactivity, autonomic hyperarousal, inflammatory disorders, disturbed sleep, and dysregulated hypothalamic-pituitary-adrenocortical activity, also exacerbate CVD risk (Friedman and McEwen 2004; O’Toole and Catts 2008; Moazen-Zadeh et al. 2016). PTSD sufferers also often exhibit psychological conditions and behavioral patterns (e.g., vital exhaustion, poor coping, substance abuse and other risk behaviors), that further increase CVD risk (Buckley et al. 2004; Dedert et al. 2010).
Gender, Stress Exposure and CVD
The stress-CVD relationship is complex and stress does not cause CVD in a singular, undifferentiated pathway. Understanding gender differentiation in the association between wartime stress exposure and CVD is critical, as women increasingly enter armed forces globally, and as armed conflict increasingly affects noncombatants. While the war-related trauma-PSTDCVD pathway has been understudied in women, several studies suggest that PTSD is a critical CVD risk factor for both men and women (Sumner et al. 2015, 2016; Kibler et al. 2018; Kubzansky et al. 2009). Yet quite few studies to our knowledge explicitly test whether gender moderates the pathway linking psychosocial stressors to later life CVD (e.g., Batten et al. 2004; von Känel 2012; Matheson et al. 2010). Gender-moderated associations between stress exposure and CVD (as well as inflammatory activation) suggest a role for gender socialization, as well as men’s and women’s differentiated responses to caregiver stress and other stressors associated with gender-linked social roles (e.g., Shivpuri et al. 2012; Matheson et al. 2009). In contexts of conflict, young men may be socialized to expect to encounter violence, and to serve in military roles and armed conflict, expectations that do not usually extend to young women (Axinn et al. 2013; Eichler 2011). Differential socialization around participation in violence and conflict may result in distinctive health consequences for men and women who experience similar degrees of war-related stress exposure.
In addition to the war-related stressors of their adolescence and early adulthood, and the stressors of economic volatility following in the wake of late twentieth century market reforms, older Vietnamese experience myriad life event stressors that are associated with the life course of aging, such as widowhood, the death of family members, and caregiving for severely ill kin, which also demonstrate an association with CVD (e.g., Capistrant et al. 2012). Furthermore, and more so than is common in many western populations, wide swathes of older adults in LMICs also endure acute and chronic stressors linked to negative income shocks and persistent deficits in their material conditions (Liebenehm 2018). In assessing associations among war-related stressors, PTSD and CVD it is also important to account for commonplace stressors in the daily lives of Vietnamese older adults which may also exacerbate CVD risk.
We illustrate our conceptual model for wartime stress exposures and CVD in Figure 1.
Figure 1.
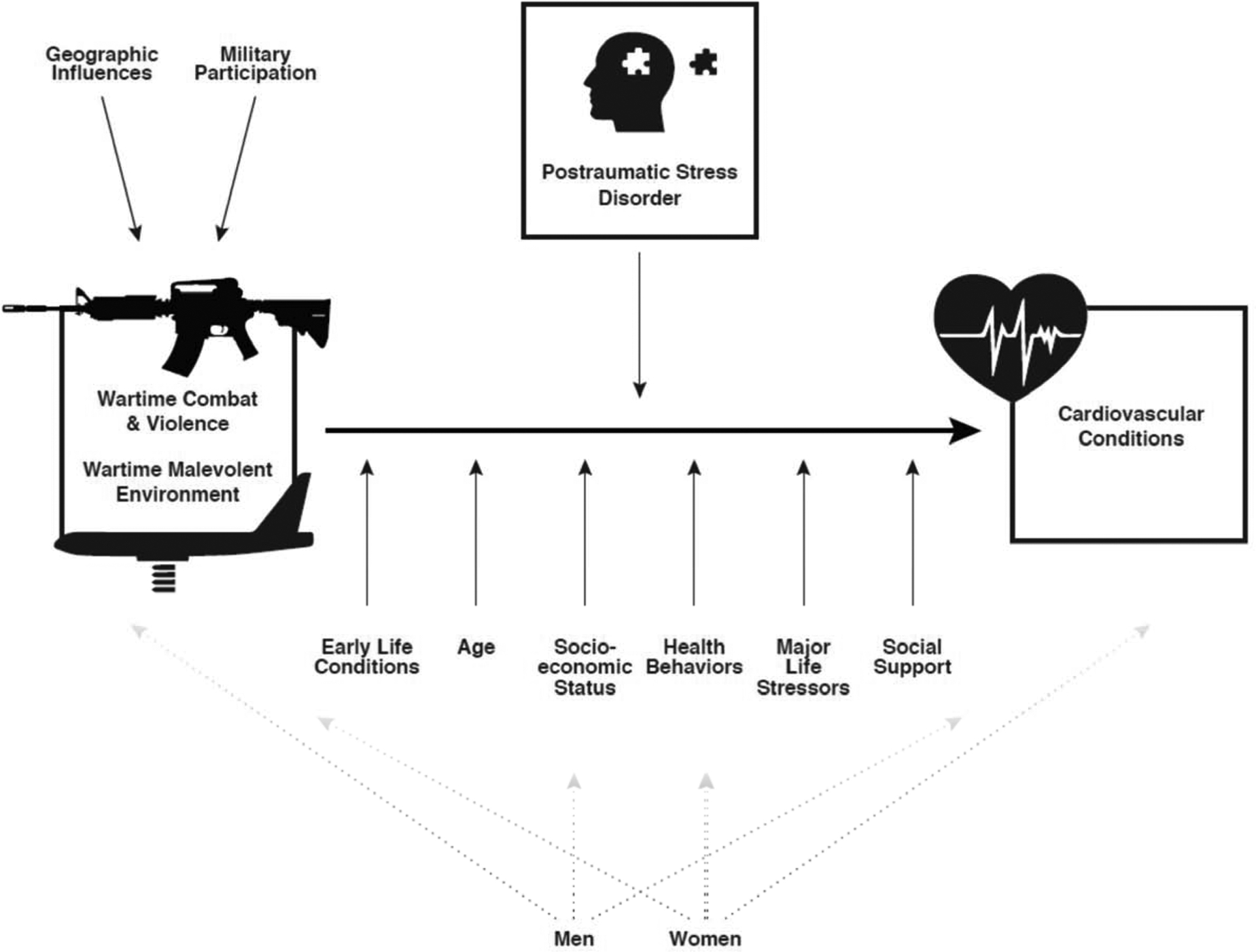
Model of War-related Stress and Cardiovascular Conditions
Vietnam’s American War, Stress Exposure, and Health among Aging Survivors
Vietnam’s American War exacted a severe toll upon Vietnamese society (Hirschman et al. 1995), yet knowledge of its enduring impacts still derives largely from American soldiers’ perspectives. Vietnam’s geopolitical context, historically high war mobilization, and movement of war survivors into older ages where health and life course transitions are common, make it a valuable case for studying war’s long-term impacts (Van Dyke 1972; Pike 1986). Certain Vietnamese survivors of its war (1965–75) were directly involved in concentrated wartime activities, having served in the military, lived through devastating combat, and lost family members violently. Others were less directly involved, living at a distance or on the periphery of war zones. These divergent experiences provide a rare opportunity for investigation.
Not an experience limited to soldiers, citizens of Vietnam broadly experienced war in their midst, and both soldiers and civilians faced diverse stressors, from ambushes and near misses in firefights, to forced evacuation and exposure to the dying and wounded (Young et al. unpublished results). The American War in Vietnam brought many women near to the front, while others witnessed war violence visited upon their family members, homes and communities (Turner-Gottschang and Phan 1998). Accordingly, Vietnam provides a critical setting to understand gendered exposure to war-related stressors and war’s gender differentiated health consequences. Variants of the malevolent environmental conditions or daily hassles that correlate with PTSD among American veterans of the Vietnam War, such as resource insufficiencies and harsh living conditions, were commonplace for Vietnamese, not only among soldiers but also among militia volunteers and many civilians (Fontana and Rosenheck 1999). In addition to soldiers in formal military units, the government of the Democratic Republic of Vietnam also mobilized the Thanh Niên Xung Phong, or Youth Shock Brigades (YSB), a volunteer force or ‘reservist’ army that engaged many rural youths in their teens and early twenties, whose duties brought them close to the fighting, to provision arms and foodstuffs, defuse bombs on the front lines, and maintain roads and the Ho Chi Minh trail (Guillemot 2009:23). The efforts of these youth brigades, many of whom were women, were crucial to the war effort, yet they are not enumerated as veterans of the North Vietnamese Army.
We conduct this investigation using a novel dataset collected among a cohort of Vietnamese older adults who experienced wide ranging exposures to wartime stress when study participants were in their adolescence and early adulthood. In the cohort of war survivors that we analyze, war-related stressors ranged from chronic to acute, for over a decade of time, among military service members and civilians. The present, population-based study also permits analysis of individuals who spanned formal military roles, service in the YSB and various militia organizations, and civilians who did not engage in military activities but who, nonetheless, experienced myriad stressors due to their proximity to the fighting and family members’ involvement in the fighting. War took place in and among communities in which people lived, yet there was substantial community-level variation in exposures. Thus, Vietnam not only offers a different milieu for studying the impact of stress on later life CVD as compared to past studies; the wide-ranging variation in stress exposures also provides a type of natural experimental design.
Hypotheses:
We hypothesize that CVD conditions will be greater among older adults who experienced greater levels of wartime stress exposure (H1). We further hypothesize that exposure to both the stressors of combat and proximity to violent death, as well as wartime malevolent environment stressors, will each exhibit significant associations with late-life CVD conditions (H2). Given the physiological and psychological pathologies shown to accompany PTSD, we hypothesize that the associations between war-related stress and CVD conditions will be largely mediated by recent experience of PTSD (H3). Finally, we hypothesize that the association between war-related stressors and CVD will be moderated by gender, such that women with high levels of war-related stress exposure will experience more greatly heightened odds of CVD conditions relative to their male counterparts (H4).
Data and Methods
This study is based upon analyses of the Vietnam Health and Aging Study (VHAS). The VHAS is an international collaborative research study, funded by the National Institutes of Health/National Institute on Aging (R01 AG052537), that allows for investigation of the mechanisms of association between diverse exposures to armed conflict during the Vietnam War and multiple dimensions of health among older adults.
The VHAS data collection was conducted with 2,447 Vietnamese adults age 60 and older in order to examine life course, social relational, and health and mortality transitions among a population of war survivors. Working together with commune and district level population volunteers, VHAS staff sampled prospective participants from four northern Vietnamese districts. These districts were purposively chosen as study sites to capture a wide spectrum of regionally-based war exposure as indicated by intensity of bombings. Within each district, VHAS staff randomly selected two wards or four communes to arrive at a total of 12 primary sampling units (PSUs). Individuals were then selected for study participation from household registration systems maintained within PSUs using stratified, random sampling within four subdomains of interest: male-veteran, male-nonveteran, female-veteran, female-nonveteran. Selected individuals were contacted by phone or in person to recruit their participation. Among those agreeing to participate, project staff scheduled an appointment for the in-person interview and biomarker collection. A small cash payment and basic health check by a local health worker were provided as incentive for participation. If the selected individual refused to participate, a replacement individual was randomly selected from the commune or ward household roster. The overall response rate for the VHAS Wave I data collection was 84.7%. The refusal rate among selected participants who then failed to complete the interview or biomarker collection for some reason was 9.7% overall.
VHAS survey data collection, taking place over the months of May to August, 2018, entailed an approximately 2.5 hour face-to-face home-based interview and 30-minute biomarker collection. Interview questions and formatting were developed, pretested, and programmed with the COMMCARE mobile data collection platform onto tablet PCs over the course of several months prior to data collection. The interview focused upon numerous dimensions of military service, wartime experiences, health, healthcare, material conditions, household composition, spousal and intergenerational relationships, migration history, and social support and engagement, among others. Full or partial proxy interviews were conducted with sampled individuals who were too frail or cognitively impaired to perform the interview independently. In addition to individual interviews, the VHAS conducted clinic-based biomarker collection with all respondents. Twelve commune health centers hosted biomarker data collection, conducted by trained VHAS staff on the day following the VHAS interview. VHAS conducted body measurements, functional tests, finger-prick capillary blood collection, and hair collection. The resultant biomarkers allow for estimation of the biological pathways linking traumatic stress, chronic stress, health outcomes and physiological aging. Overall, participation in the biomarker collection component of the study was high. Nearly 96% of participants agreed to participate in the biomarker data collection. We utilize the VHAS field-based blood pressure measurements to construct the measure of hypertension in the current study. A more detailed description of the VHAS procedures is elaborated elsewhere (Korinek et al. 2019).
Variable measurement
Our outcome variables include four cardiovascular conditions, two of which constitute prime risk factors for CVD (hypertension and dyslipidemia) and two of which are cardiovascular diseases representing leading causes of mortality and disability worldwide (heart disease and stroke) (Wajngarten and Silva 2019). We assess hypertension using the average of two consecutive blood pressure measurements. We designate as hypertensive those with Stage 2 hypertension, i.e., with systolic blood pressure reading of >=140 and/or diastolic blood pressure reading of >89 (Whelton et al. 2018), and those with lower blood pressure readings who indicate they are presently taking medication for hypertension. For the latter three conditions, dyslipidemia, heart disease, and stroke, we construct binary indicators based upon respondents’ self-reports of whether or not they had the condition. We count both self-reported conditions diagnosed by doctors, and those conditions respondents indicate are present but not diagnosed by a doctor. The vast majority of respondents indicate the conditions have been diagnosed by a doctor.
Our focal explanatory variables assess exposure to wartime stressors. Questions were derived from the Deployment Risk and Resilience Inventory (King et al. 2006), and the Combat Exposure Scale (Keane et al. 1989) and adapted to address the nature of war exposure in the Vietnam context. We use confirmatory factor analyses to reduce the full set of questions into two indices (Young et al. unpublished results). The first index captures combat experiences and encounters with wartime violence. This additive scale enumerates the following experiences: 1) engaging in combat patrols or dangerous duties; 2) having been ambushed; 3) engaging in a unit that fired at the enemy; 4) causing the death of an enemy combatant; 5) experiencing a near miss of being shot; 6) witnessing a friend/comrade shot in battle; 7) being exposed to toxic chemicals during the war 8) being wounded as a result of the war; 9) seeing dead Vietnamese soldiers; 10) seeing dead American soldiers; 11) seeing dead civilians, and 12) knowing someone seriously injured or killed in war. As they apply to those who served in military or military-like roles, items 1–6 were asked only of respondents who served in the formal military, militia organizations or the YSB. Others with no reported military participation are assumed to have experienced no exposure of the kind represented in items 1–6, and thus are coded zero on these items.
Next, we adapted Fontana and Rosenheck’s (1999) approach to assess wartime malevolent living conditions. Items in this additive scale include: 1) having had to move as a result of the bombing of one’s home/village; 2) having had to evacuate (as a precursor to expected bombing or other attack); 3) experiencing a lack of clean water; 4) experiencing food shortage that caused illness or weakness; 5) experiencing difficulty sleeping due to noise or inhospitable surroundings. The first two items, which capture forced displacement as a result of the war, represent additions to Fontana and Rosenheck’s instrument. Forced displacement is a salient event, frequently faced by civilians living within or near warzones, associated with myriad stressors.
The VHAS questionnaire assessed the frequency or severity of each item in the war stress exposure scales. However, to derive the scales in this study we recoded each item to indicate whether the respondent experienced each item ever (coded as one), or never (coded as zero). These binary coded items are then summed to derive two summative exposure indices: Wartime Combat and Violence (range: 0–12), and Wartime Malevolent Environment (range: 0–5).
The assessment of PTSD in VHAS is based upon participants’ self-reported experience of a subset of symptoms drawn from the 20-item Posttraumatic Checklist (PCL-5). Specifically, all VHAS respondents were asked to indicate whether they had experienced ten specific forms of stress (e.g., disturbing or unwanted memories of war experience; irritable/aggressive behavior) in relationship to “the most intense or stressful event(s)” they experienced during the war and how much the symptom(s) had bothered them (not at all, a little, moderately, a lot) in the past year. 1 The VHAS PTSD questions were reduced to ten in order to minimize respondent burden within a lengthy questionnaire instrument, and to remove questions that, during pretesting, prove to translate poorly, either linguistically or culturally, to the Vietnam context, or were politically sensitive. Questions tapped the respondents’ level of re-experiencing traumatic events, avoiding reminders, emotional numbing, arousal, and anxiety. In the current study we create a continuous variable based on the sum of severity scores for each PSTD item.
We also consider modes of lifetime forms of military service alongside our measures of wartime stress exposures. Military service may have been formal service in the North Vietnamese Army, or it may have been informal, performed in a militia unit or the YSB (Guillemot 2009). Accordingly, military service status is coded as formal military, militia/youth shock brigade, or no military service. Our data suggest men and women who performed militia/YSB service experienced wartime stressor levels intermediate those of soldiers in the formal military and civilians.
In order to assess how recent live event stressors correlated with CVD outcomes, alongside the more ‘remote’ wartime stressors, we constructed a summative index capturing respondents’ self-reported experience of six life event stressors within the past 2–3 years: an accident that caused physical or psychological injury, marital disruption (widowhood or divorce), a major residential move, the death of a child, the severe illness of a spouse, or severe financial difficulty.
We include a set of control variables in all analyses. These include: sex; current age; educational attainment (whether the respondent attained secondary or higher education, or lower levels of education); main lifetime occupation (measured categorically as Agricultural occupation, Professional/technical occupation, or Other occupation); household asset ownership (a PCA-weighted index of the following items: television, telephone, refrigerator, air conditioner, motorbike, automobile, computer, home internet access); self-reported childhood health status; smoking status (whether never smoked, formerly smoked, currently smoke); current alcohol consumption (none, < 1 drink per day, 1–2 drinks per day, > 2 drinks per day). We also include as a control variable the district of residence. The district context may represent a number of characteristics salient to CVD outcomes, including the current level of urbanization, economic development and the degree of bombing exposure during the war (Miguel and Roland 2011).
Analytical Approach
We proceed with bivariate (Table 1) and multivariate analyses of current cardiovascular conditions (Table 2). In multivariate analyses we utilize survey-adjusted logistic regression modeling in Stata version 15.1. This approach addresses features of the survey design and randomized selection of respondents within commune-level primary sampling units. We present two models for each of the four CVD outcomes. The first model includes the independent variables and all covariates except for PSTD symptoms. PTSD symptoms are added to the second set of models as a means to assess PTSD as a mediating pathway via which wartime stress exposures indirectly influence CVD. To further assess PTSD as a mediating variable within the logit models estimating binary CVD outcomes, we utilize the ldecomp command within Stata to decompose the direct effects of our stress exposure indices and their indirect effect upon CVD via PTSD (Buis 2010).
Table 1.
Descriptive Statistics, Vietnam Health and Aging Study (VHAS) (unweighted data)
| Total Sample | Male Subsample | Female Subsample | ||||
|---|---|---|---|---|---|---|
| %/Mean | N | %/Mean | N | %/Mean | N | |
| Cardiovascular Disease Outcomes | ||||||
| Cardiovascular Conditions | ||||||
| Dyslipdemia | 20.7 | 460 | 21.0 | 228 | 20.4 | 232 |
| Hypertension | 59.4 | 1,396 | 63.9 | 735 | 55.1 | 661 |
| Heart Condition | 23.1 | 549 | 24.5 | 286 | 21.8 | 263 |
| Stroke | 7.5 | 181 | 10.0 | 118 | 5.1 | 63 |
| Mean count of Cardiovascular Conditions | 1.10 | 2,100 | 1.18 | 1,033 | 1.02 | 1,067 |
| Wartime Stressors | ||||||
| Wartime Exposure to Combat and Violence Scale (0–11) | 2.8 | 2,422 | 4.6 | 1,182 | 1.11 | 1.240 |
| Wartime Malevolent Living Conditions Scale (0–5) | 1.4 | 2,446 | 1.5 | 1,195 | 1.31 | 1,251 |
| Recent PTSD Symptoms | 5.3 | 2,447 | 5.64 | 1,195 | 4.91 | 1,252 |
| Recent Life Event Stressors | ||||||
| Sum of Recent Life Event Stressors | 0.93 | 2,441 | 0.89 | 1,193 | 0.971 | 1,248 |
| Control Variables | ||||||
| Age (mean) | 70.3 | 2,445 | 69.0 | 1,194 | 70.81 | 1,251 |
| Sex | ||||||
| Male | 48.8 | 1,195 | 100.0 | 1,195 | 0.0 | 0 |
| Female | 51.2 | 1,252 | 0.0 | 0 | 100.01 | 1,251 |
| Health in Childhood (fair/poor/very poor) | 35.8 | 840 | 33.1 | 384 | 38.5 | 456 |
| Health in Childhood (good/very good) | 64.2 | 1,506 | 66.9 | 776 | 61.5 | 730 |
| Education: Less than Secondary | 81.4 | 1,984 | 72.8 | 868 | 89.71 | 1,116 |
| Education: Secondary or higher | 18.6 | 453 | 27.2 | 325 | 10.3 | 128 |
| Main Lifetime Occupation: Agriculture | 67.1 | 1,643 | 58.7 | 702 | 75.2 | 941 |
| Main Lifetime Occupation: Professional, Technical | 11.9 | 292 | 14.7 | 176 | 9.3 | 116 |
| Main Lifetime Occupation: Other | 20.9 | 512 | 26.5 | 317 | 15.6 | 195 |
| Weighted Index of HH Assets | 2.28 | 2,445 | 2.40 | 1,194 | 2.161 | 1,251 |
| Smoking status: Never | 18.5 | 221 | 97.91 | 1,226 | ||
| Smoking status: In past | 29.9 | 357 | 0.6 | 7 | ||
| Smoking status: Current smoker | 51.6 | 617 | 1.5 | 19 | ||
| Avg Daily Alcohol consumption: None | 30.2 | 360 | 86.1 | 1,076 | ||
| Avg Daily Alcohol consumption: LT 1/day | 43.5 | 519 | 12.4 | 155 | ||
| Avg Daily Alcohol consumption: 1–2/day | 19 | 227 | 1.4 | 17 | ||
| Avg Daily Alcohol consumption: GT 2/day | 7.4 | 88 | 0.2 | 2 | ||
| Family relationship support index (0–4) | 1.03 | 2,396 | 0.99 | 1,191 | 1.071 | 1,205 |
| Not presently married (ie widowed, divorced) | 28.4 | 695 | 7.5 | 90 | 48.4 | 605 |
| Currently married | 71.6 | 1,751 | 92.5 | 1,105 | 51.6 | 646 |
| District of Residence | ||||||
| Low intensity of wartime bombing | 33.4 | 816 | 33.0 | 394 | 33.7 | 422 |
| Moderate intensity of wartime bombing | 33.4 | 816 | 33.5 | 401 | 33.2 | 415 |
| Moderate-high intensity of wartime bombing | 16.6 | 407 | 16.8 | 201 | 16.5 | 207 |
| High intensity of wartime bombing | 16.7 | 408 | 16.7 | 199 | 16.6 | 208 |
Source: Vietnam Health and Aging Study, Wave I
Table 2.
Survey-adjusted Logistic Regression Results (Odds Ratios): Cardiovascular Conditions and War-related Experiences, Vietnamese Older Adults Age 60+, 2018
| Predictors | Dyslipidemia | Hypertension | ||||||
|---|---|---|---|---|---|---|---|---|
| Model Ai | Model Aii | Model Bi | Model Bii | |||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| War-related experiences & PTSD | ||||||||
| Wartime Combat and Violence Stress Exposure Index | 1.057** | [1.018,1.098] | 1.037 | [0.988,1.089] | 1.014 | [0.974,1.055] | 1.013 | [0.978,1.049] |
| Wartime Malevolent Environment Conditions Index | 1.014 | [0.936,1.098] | 0.999 | [0.928,1.075] | 1.018 | [0.922,1.124] | 1.017 | [0.917,1.129] |
| Served in formal military (ref: no military service) | 1.025 | [0.597,1.760] | 1.04 | [0.608,1.780] | 0.846 | [0.684,1.045] | 0.846 | [0.685,1.045] |
| Served in militia/ysb (ref: no military service) | 1.463* | [1.048,2.042] | 1.446* | [1.033,2.024] | 1.037 | [0.854,1.259] | 1.036 | [0.855,1.256] |
| Recent Life Event Stressors | 1.197** | [1.085,1.321] | 1.163** | [1.049,1.290] | 0.98 | [0.843,1.139] | 0.978 | [0.841,1.137] |
| Recent PTSD Symptoms | 1.029* | [1.002,1.056] | 1.001 | [0.979,1.024] | ||||
| Control Variables | ||||||||
| Age | 0.967** | [0.949,0.987] | 0.968** | [0.949,0.987] | 1.046*** | [1.027,1.066] | 1.046*** | [1.027,1.066] |
| Female (ref: Male) | 1.23 | [0.785,1.927] | 1.211 | [0.794,1.846] | 0.692+ | [0.468,1.024] | 0.691 + | [0.468,1.020] |
| Ninh Binh district (ref: Bavi district) | 1.889*** | [1.459,2.445] | 1.856*** | [1.441,2.391] | 1.557* | [1.012,2.397] | 1.556* | [1.012,2.392] |
| Bo Trach district | 1.147 | [0.856,1.536] | 1.123 | [0.857,1.471] | 1.249 | [0.718,2.174] | 1.248 | [0.721,2.159] |
| Dong Hoi town | 0.633* | [0.430,0.932] | 0.629* | [0.423,0.936] | 1.043 | [0.690,1.577] | 1.043 | [0.692,1.572] |
| Health in Childhood (fair/poor/very poor) (ref: good/v. good) | 1.101 | [0.918,1.322] | 1.115 | [0.933,1.333] | 1.136* | [1.031,1.252] | 1.137* | [1.035,1.248] |
| Education: Secondary or higher (ref: LT secondary) | 1.271 + | [0.959,1.684] | 1.271+ | [0.969,1.666] | 1.223 | [0.906,1.652] | 1.223 | [0.905,1.653] |
| Main Lifetime Occupation: Professional, Technical (ref: agriculture) | 1.860* | [1.197,2.891] | 1.870** | [1.222,2.860] | 1.212 | [0.812,1.807] | 1.212 | [0.812,1.808] |
| Main Lifetime Occupation: Other (ref: agriculture) | 1.599* | [1.055,2.422] | 1.603* | [1.059,2.428] | 1.078 | [0.774,1.502] | 1.078 | [0.773,1.503] |
| Weighted Index of HH Assets | 1.302*** | [1.170,1.448] | 1.308*** | [1.169,1.463] | 0.888+ | [0.776,1.015] | 0.888+ | [0.775,1.017] |
| Family relationship support index | 0.923 | [0.663,1.283] | 0.901 | [0.651,1.248] | 0.851 | [0.601,1.207] | 0.85 | [0.601,1.203] |
| Currently married (ref: not currently married) | 1.023 | [0.679,1.543] | 1.019 | [0.683,1.521] | 0.887 | [0.624,1.262] | 0.887 | [0.624,1.262] |
| Smoking status: In past (ref: Never smoked) | 0.834 | [0.603,1.152] | 0.849 | [0.599,1.203] | 0.866 | [0.555,1.354] | 0.867 | [0.554,1.357] |
| Smoking status: Current smoker (ref: Never smoked) | 1.27 | [0.801,2.013] | 1.287 | [0.802,2.066] | 1.23 | [0.765,1.978] | 1.231 | [0.765,1.982] |
| Avg Daily Alcohol consumption: LT 1/day (ref: None) | 0.829 | [0.589,1.165] | 0.83 | [0.588,1.173] | 0.961 | [0.795,1.162] | 0.961 | [0.795,1.162] |
| Avg Daily Alcohol consumption: 1 −2/day (ref: None) | 0.629+ | [0.367,1.078] | 0.619+ | [0.365,1.050] | 1.151 | [0.661,2.003] | 1.15 | [0.659,2.006] |
| Avg Daily Alcohol consumption: GT 2/day (ref: None) | 0.6 | [0.264,1.361] | 0.599 | [0.268,1.341] | 1.029 | [0.589,1.799] | 1.029 | [0.588,1.801] |
| Constant | 0.401 | [0.059,2.723] | 0.363 | [0.056,2.371] | 0.062** | [0.010,0.401] | 0.062** | [0.009,0.404] |
| N | 2082 | 2082 | 2198 | 2198 | ||||
| Predictors | Heart disease | Stroke | ||||||
| Model Ci | Model Cii | Model Di | Model Dii | |||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| War-related experiences & PTSD | ||||||||
| Wartime Combat and Violence Stress Exposure Index | 1.033 | [0.979,1.089] | 0.993 | [0.929,1.060] | 1.076+ | [0.995,1.163] | 1.043 | [0.958,1.135] |
| Wartime Malevolent Environment Conditions Index | 1.090* | [1.007,1.181] | 1.057 | [0.980,1.140] | 0.917 | [0.734,1.145] | 0.892 | [0.705,1.128] |
| Served in formal military (ref: no military service) | 0.813 | [0.513,1.289] | 0.839 | [0.529,1.332] | 0.595 + | [0.341,1.038] | 0.613 + | [0.348,1.078] |
| Served in militia/ysb (ref: no military service) | 1.1 | [0.761,1.590] | 1.087 | [0.775,1.524] | 0.703 | [0.362,1.365] | 0.707 | [0.362,1.380] |
| Recent Life Event Stressors | 1.307*** | [1.221,1.399] | 1.234*** | [1.145,1.330] | 1.407*** | [1.195,1.657] | 1.338** | [1.156,1.550] |
| Recent PTSD Symptoms | 1.060*** | [1.033,1.088] | 1.048* | [1.004,1.093] | ||||
| Control Variables | ||||||||
| Age | 0.99 | [0.968,1.012] | 0.991 | [0.970,1.013] | 1.027+ | [0.998,1.056] | 1.029+ | [0.998,1.060] |
| Female (ref: Male) | 0.721 | [0.441,1.179] | 0.692 | [0.419,1.143] | 0.436+ | [0.190,1.003] | 0.418* | [0.183,0.950] |
| Ninh Binh district (ref: Bavi district) | 1.680* | [1.161,2.430] | 1.626* | [1.139,2.323] | 2.038*** | [1.439,2.885] | 1.985** | [1.402,2.809] |
| Bo Trach district | 1.088 | [0.730,1.622] | 1.034 | [0.717,1.489] | 2.129** | [1.267,3.577] | 2.035* | [1.189,3.484] |
| Dong Hoi town | 0.756 | [0.506,1.129] | 0.737 | [0.506,1.076] | 2.107* | [1.103,4.023] | 2.087* | [1.089,4.001] |
| Health in Childhood (fair/poor/very poor) (ref: good/v. good) | 0.993 | [0.829,1.190] | 1.015 | [0.845,1.217] | 1.328* | [1.083,1.628] | 1.345** | [1.096,1.650] |
| Education: Secondary or higher (ref: LT secondary) | 0.875 | [0.592,1.293] | 0.871 | [0.583,1.303] | 0.577 | [0.283,1.174] | 0.583 | [0.292,1.166] |
| Main Lifetime Occupation: Prof’l, Technical (ref: agric) | 1.851** | [1.317,2.601] | 1.884** | [1.333,2.662] | 1.903 + | [0.881,4.109] | 1.949+ | [0.883,4.302] |
| Main Lifetime Occupation: Other (ref: agric) | 1.617** | [1.214,2.153] | 1.642** | [1.243,2.169] | 1.491 | [0.910,2.441] | 1.509 | [0.901,2.527] |
| Weighted Index of HH Assets | 0.845+ | [0.696,1.026] | 0.856+ | [0.712,1.028] | 1.033 | [0.713,1.495] | 1.042 | [0.714,1.522] |
| Family relationship support index | 0.948 | [0.754,1.193] | 0.912 | [0.717,1.159] | 0.945 | [0.520,1.717] | 0.919 | [0.493,1.713] |
| Currently married (ref: not currently married) | 0.919 | [0.674,1.252] | 0.913 | [0.663,1.259] | 1.01 | [0.553,1.845] | 0.989 | [0.536,1.827] |
| Smoking status: In past (ref: Never smoked) | 0.896 | [0.538,1.493] | 0.937 | [0.558,1.575] | 1.029 | [0.511,2.074] | 1.075 | [0.541,2.136] |
| Smoking status: Current smoker (ref: Never smoked) | 1.380+ | [0.991,1.921] | 1.439* | [1.038,1.994] | 1.674 | [0.771,3.634] | 1.749 | [0.826,3.704] |
| Avg Daily Alcohol consumption: LT 1/day (ref: None) | 0.642** | [0.470,0.877] | 0.630** | [0.456,0.870] | 0.387*** | [0.256,0.583] | 0.381*** | [0.256,0.568] |
| Avg Daily Alcohol consumption: 1 −2/day (ref: None) | 0.656 | [0.389,1.107] | 0.623 + | [0.383,1.016] | 0.450* | [0.231,0.875] | 0.430* | [0.222,0.835] |
| Avg Daily Alcohol consumption: GT 2/day (ref: None) | 0.511 | [0.220,1.184] | 0.503 + | [0.218,1.161] | 0.619 | [0.261,1.471] | 0.62 | [0.263,1.461] |
| Constant | 0.628 | [0.060,6.571] | 0.488 | [0.048,4.921] | 0.003*** | [0.000,0.051] | 0.002*** | [0.000,0.043] |
| N | 2223 | 2223 | 2262 | 2262 | ||||
p <0.10,
p < 0.05,
p < 0.01,
p < 0.001
Source: Vietnam Health and Aging Study (VHAS), 2018
Results
Descriptive Statistics
Descriptive statistics for the analytical sample are shown in Table 1. Cardiovascular conditions are prevalent in the older adult VHAS sample comprised of men and women age 60 and older. Each respondent reports experiencing, on average, one of the four conditions measured in the study.
Especially high is hypertension, with nearly 60% of the sample having hypertension as assessed in the VHAS clinic-based data collection. Over one-fifth of the VHAS sample self-reports high cholesterol (20.7%) and heart disease (23.1%), while 7.5% self-report stroke. As observed in past studies (Son et al. 2012; Tran et al. 2018), a greater share of men than women experience each of these CVD conditions, and the gender difference is statistically significant (Pearson chi-square Pr = .000) for the prevalence of stroke and hypertension.
Considering exposure to wartime combat and violence, respondents experienced, on average, approximately 2.8 of the enumerated war-time stressors, with men reporting significantly higher exposures to wartime stressors of this nature. Exposures to wartime malevolent conditions averaged 1.4, with men and women reporting similar levels of exposure to stressors of this kind.
On a scale from zero to six, respondents experienced 0.9 of the enumerated recent life event stressors over the past 2–3 years on average. Most common were great financial hardship and the severe illness of a spouse.
Table 1 further indicates that just slightly over half of the VHAS sample is female (51.2%), their median age is 70, and 18.6% have some secondary or higher levels of education. The majority have labored in agriculture as their main lifetime occupation. Just over one-third (35.3%) report having had fair, poor or very poor health in childhood, and while alcohol consumption is relatively low in the sample overall, current and former smoking status is relatively high, but only in the male subsample. Over 50% of men indicate they are current smokers and nearly one-third are former smokers. Analyses of the VHAS pilot study found that lifetime smoking is positively associated with military service in this cohort of Vietnamese older adults (Korinek et al. 2017).
Multivariate Analysis Results
The results of survey-adjusted logistic regression models estimated each of four CVD conditions are presented side-by-side in Table 2. For each CVD condition we estimate two models, the second incorporating recent PTSD symptoms along with the war-time stressors and other covariates. Odds ratios are presented, along with 95% confidence intervals.
As results in Table 2 demonstrate, in two of the four survey-adjusted logistic regression analyses we observe a statistically significant, positive association between war-related stress exposure and CVD conditions, in particular dyslipidemia and heart disease. For a third condition (stroke) we observe a result that is marginally significant and positive. For stroke and its common risk factor, dyslipidemia, the magnitude of exposure to wartime combat and violence is a significant, positive predictor. For heart disease, exposure to wartime malevolent environment stressors is a significant predictor. These results provide partial support for our first hypothesis concerning early life wartime stress exposure and CVD in late adulthood. The mixed results suggest it is important to identify and explore those specific health conditions, even within the cardiovascular health domain, that are most impacted by war-related stressors.
The results in Table Two are not entirely consistent with our second hypothesis. In particular, we hypothesized that both types of stressors, combat and proximity to violent death, as well as wartime malevolent environment stressors, would each correlate with late-life CVD conditions. Rather, we observe a more complex, differentiated pattern. Although we initially conceived of particularly poor cardiovascular consequences resulting from the severe, often acute, stressors of combat, such as being fired upon or witnessing death at close range, for certain CVD outcomes (i.e., heart disease), it is only the exposure to malevolent living conditions during wartime that exhibits a statistically significant association with CVD and CVD risks. These differential patterns of association between specific stressor types and specific CVD conditions remain when we estimate models containing only one war stress exposure type, either malevolent environment or combat/violence expsosures.
As the second model for each of our CVD conditions demonstrates, the association between war-related stress exposure and CVD is largely expressed through recent PTSD symptoms. Recent PTSD symptoms are statistically significant in three of the four models, and their inclusion reduces the war-time stress exposure odds ratios to statistical insignificance across all three outcomes. In particular, for dyslipidemia the significant positive association with combat and violence stress exposure in Model Ai (OR=1.057, p<=.01) is reduced to insignificance in Model Aii (OR=1.037, n.s.) with the inclusion of the recent PTSD symptom count (OR=1.029, p<=.01). Similarly, for heart disease the significant positive association with malevolent environment exposures in Model Ai (OR=1.090, p<=.05) is reduced to insignificance in Model Aii (OR=1.057, n.s.) with the inclusion of the recent PTSD symptom count (OR=1.060, p<=.001). An analogous pattern is also observed for stroke with the inclusion of recent PTSD symptoms. This pattern of results indicates that the psychological response to wartime stressors is a key pathway through which war-related stress exposure influences later life CVD conditions. We also utilize ldecomp in Stata to decompose the direct and indirect effects of wartime stress exosure within the three logit models which demonstrate statistically significant assocations with particular CVD outcomes (see Supplementary Tables). These results confirm that war stress exposure’s effects upon CVD outcomes are largely mediated through recent PTSD symptoms. This pattern of results is consistent with much past research and with our third hypothesis.
Gender interaction models and marginal effects
In the models shown in Table 2, hypertension appears to exhibit no association with war-related stress exposures or PTSD. However, side-by-side analyses of male and female subsamples (available in Supplementary Tables) are indicative of a significant, positive association between war-related stress exposure and hypertension among women, but not men. Accordingly, we ran three separate logistic regression models predicting hypertension, each including an interaction term between respondent gender and the following variables: Index of Exposure to Combat and Violence, Index of Exposure to Malevolent Environment Conditions, and Recent Count of PTSD Symptoms, respectively. In the models predicting hypertension the gender interaction terms and marginal effects were statistically significant for both of the war-related stress exposure indices, but not the interaction with PTSD. We show these results in Figures 2A and 2B. We do not observe statistical significance for the gender and war-related stress exposure indices in models predicting the other three CVD conditions.
Figure 2A.

Predictive Margins of Sex and Index of Exposure to Combat and Death (with 95% CIs)
Figure 2B.
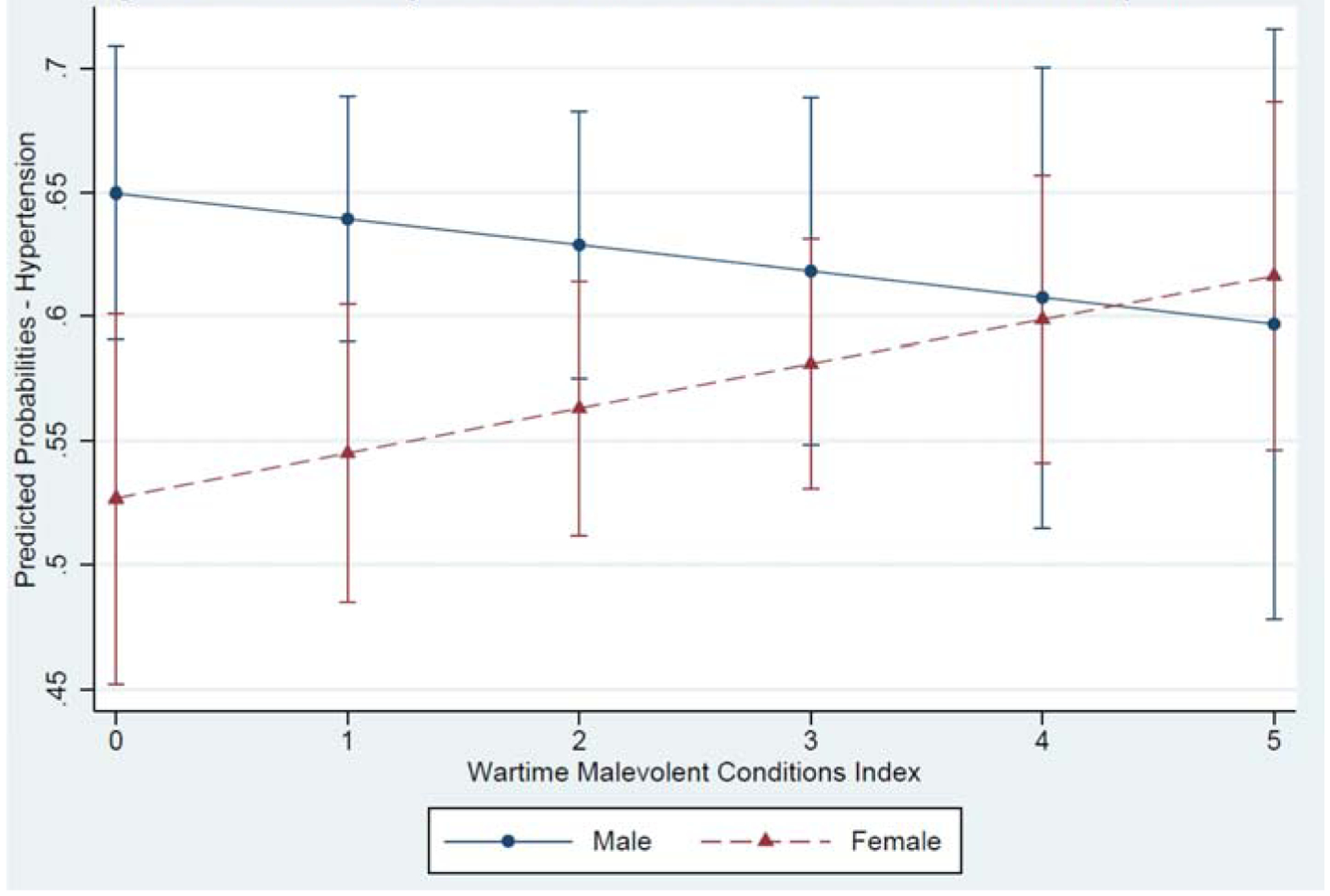
Predictive Margins of Sex and Index of Wartime Malevolent Conditions Exposure
Notably, we observe that among men there is a modest, negative assocation between war-related exposure to combat and violence, and wartime malevolent environment conditions, and the probability of hypertension. For women, however, the pattern of association is reversed. Thus, while at low levels of exposure men’s risk of hypertension is significantly greater than that of women, at higher levels of exposure women’s risk is on par with that of men. In this sample of war survivors, then, men with extensive war-related stress exposure in their personal history are not so different than their male counterparts who were shielded from multiple war-related stressors in combat or in the general wartime environment. For women, though, those with multitudes of war-related stressors in their early adulthood are more likely to experience hypertension than women with lesser degrees of wartime exposure. These gendered associations are partially consistent with our fourth hypothesis, demonstrating not only that both men and women experience long-term health impacts related to war exposure, but that women may experience a greater CVD penalty related to wartime stress exposures as compared to men with similar levels of stress exposure.
Discussion
This investigation provides evidence from a unique population-based survey which suggests that the stressors of war, experienced widely among a cohort of older adults who endured myriad forms of exposure to war during their young adulthood in Vietnam, exhibit a significant association with a range of cardiovascular conditions in late adulthood. These results are consistent with past research conducted largely among western populations, and military veterans in particular, which finds that the stress of combat and related PTSD results in heightened CVD risk and CVD-mortality among veterans (Beristianos et al. 2016; O’Toole and Catts 2008; Vaccarino et al. 2013). Notably, among these northern Vietnamese older adults the “remote” stressors of wartime persist in their association with CVD, independent of recent life event stressors and socioeconomic difficulties, despite the latter’s occurrence over 40 years prior to the VHAS data collection, and our necessarily selective analysis of disease outcomes among those surviving into late adulthood.
Among VHAS respondents, war-related stress exposure operates largely through the experience of PTSD symptoms to heighten the odds of experiencing dyslipidemia, heart disease and stroke in later adulthood. In this regard, our study participants, whose life histories span a diverse spectrum of war exposures and military service, exhibit associations between war exposure and CVD, mediated by PTSD, that resemble the oft-studied U.S. veteran population.
For women in the VHAS, but not men, exposure to stressors of combat and wartime violence and death also significantly increase the odds of hypertension. Such a finding suggests that, although men are concentrated on the frontlines, women bear a significant, potentially disproportionate, long-term health penalty linked to their war exposures. The absence of a significant association between wartime combat and violence stressors and hypertension among men in our sample is somewhat puzzling. However, the gendered pattern stress exposure and gendered health consequences of war-related stress exposure in our analysis is consistent with several past studies (e.g., Batten et al. 2004; von Känel 2012). There are several possible explanations for this observed gender-differentiated association between war-related stressors and hypertension. Gendered patterns of socialization, in particular men’s socialization in early adulthood may protect them from ill consequences of war and violence exposure, as they are socially prepared to expect to endure such violence and possibly to attain a sense of status and masculine accomplishment from such experiences. Moreover, men in northern Vietnam had very high rates of participation in the formal military, whereas relatively few women engaged in military service and those who did so largely engaged in less formalized positions, such as in the YSB. As a result, the male subsample reflects greater mortality selectivity, and the associations we observe are diminished by CVD-deaths occuring that precede conduct of the study. Furthermore, surviving male veterans in the sample may be relatively resilient to wartime stressors’ ill effects, having received training that provided them with enhanced coping skills and post-war benefits that protected their wellbeing. Following from previous scholars’ observations that women see a greater CVD penalty following stress in childhood and early adulthood (Batten et al. 2004), it is possible that in enduring severe stressors women, but not men, experience a diminishment of the “natural protection” against CVD imparted by hormonal and metabolic factors (Brochier and Arwidson 1998).
Further investigation on the war-related precursors of CVD, such as those which explore the role of gender, and the nature of alcohol and tobacco use as mediating factors, will allow deeper insights into the nature of war’s lasting toll upon the processes of aging and health trajectories in Vietnam and other post-conflict countries. The pathophysiological mechanisms which link stress and CVD are diverse, from behavioral adaptations to many dysregulated physiological systems (Rozanski et al. 1999). These preliminary results provide motivation for deeper investigation of the specific mechanisms whereby war in early life shapes the life course of health and aging.
While the VHAS data provide unique insights into the health consequences of war exposure in early life, several limitations associated with measurement and analysis in the current study warrant mention. First, we are aware of the limitations associated with self-reports of health conditions, especially within a context such as Vietnam where underdiagnosis is common and health care service underutilization is significant and differentiated by socioeconomic status and other factors. The singular measure within the current study that relies upon a biomarker collected in the field, hypertension, demonstrates little association with wartime stressors or recent life events stressors. This runs counter to many previous studies and calls for further investigation of biases that may differentially influence self-report versus field-based biomarker measurement strategies. Second, our measurement of chronologically “remote” early life wartime stressors may be impacted by recall bias. Such potential biases are common to studies of older adults that rely upon retrospective questions about early life experiences. Yet, we note that several major studies of health and aging, such as the Health and Retirement Study and its sister studies in China and elsewhere, have shown that survey questions about early life conditions can be successfully linked to later-life outcomes (Haas 2008; Smith et al. 2012). While confidence in the validity of our retrospective measurement of early life stressors is enhanced by scholarship suggesting that memories from early adulthood are recollected with greater accuracy than memories from other life course stages, especially memories linked to personal anchor events, such as war, we nonetheless cautiously interpret findings that may be impacted by various biases of recall (Rubin et al. 1998; Conway et al. 2005). Third, while we are confident that war-time stress exposure occurs prior to current CVD conditions in the VHAS sample, the specific timing of measured life event stressors, assessed as having occurred within the past 2–3 years, is less definitive vis-à-vis the timing of CVD condition onset. This limitation stems from the cross-sectional nature of the data at this point in time. We will be better equipped to demarcate the onset and trajectory of CVD conditions relative to life event stressors upon the receipt of a subsequent wave of linked longitudinal data among VHAS participants. Additionally, because the VHAS Wave I data did not capture reliable information on respondents’ physical activity levels, we were not equipped to construct the full Framingham CVD Risk Score.
In conclusion, analyses of Vietnam’s cohort of older adult war survivors yields new evidence on the myriad ways that armed conflict shapes individual CVD risk, and more broadly, the global burden of disease. As Jawad and colleagues (2019: 1388) have noted, scholars and clinicians must improve their understanding of CVD risk changes during and after conflict in order to better prepare and implement evidence-based health systems interventions. Vietnam, while hard hit by war for several decades in the 20th century, is not alone among LMICs undergoing struggles of economic development while also providing healthcare services to a generation of older adults whose lives were deeply affected by war. The current study takes an important step toward addressing the linkages between specific, individual-level war exposures and CVD risk, steps which permit clearer identification of groups at risk who may benefit from prevention and treatment. Given the significance of PTSD in each CVD outcome under consideration, the current study also suggests it is important to account for mental health mechanisms in models estimating CVD, especially in LMICs where scholarship on the burden of mental illness is in relative infancy. Further understanding of the mental health pathways of CVD may illuminate early preventative hybrid treatments, reducing the burden of CVD, particularly in the LMICs where CVD is currently underdiagnosed and undertreated.
Supplementary Material
Highlights.
Early life wartime stressors carry a cardiovascular disease penalty into late adulthood
Combat violence and malevolent environment stressors predict cardiovascular disease
PTSD mediates the association between stressors of war and cardiovascular disease
Vietnamese women bear the cardiovascular burden of armed conflict alongside men
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health [R01 AG052537].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VHAS also asked participants about PTSD symptoms ever experienced since the war. The lifetime and current PTSD scores are highly correlated (r=.69), with the mean score for current symptoms (5.3) significantly lower than the mean lifetime score (12.9).
Contributor Information
Bussarawan Teerawichitchainan, Department of Sociology and Centre for Family and Population Research, National University of Singapore, Singapore..
Nguyen Thi Kim Chuc, Department of Family Medicine, Hanoi Medical University, Hanoi, Vietnam.
Miles Kovnick, Department of Sociology, University of Utah, Salt Lake City, Utah, U.S.A..
Zachary Zimmer, Department of Family Studies and Gerontology, Mount Saint Vincent University, Halifax, Nova Scotia, Canada.
References
- Anand S, Bradshaw C, & Prabhakaran D (2020). Prevention and management of CVD in LMICs: why do ethnicity, culture, and context matter?. BMC medicine, 18(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axinn WG, Ghimire DJ, Williams NE, & Scott KM (2013). Gender, traumatic events, and mental health disorders in a rural Asian setting. Journal of health and social behavior, 54(4), 444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten SV, Aslan M, Maciejewski PK, & Mazure CM (2004). Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. Journal of Clinical Psychiatry, 65(2), 249–254. [DOI] [PubMed] [Google Scholar]
- Beristianos MH, Yaffe K, Cohen B, & Byers AL (2016). PTSD and risk of incident cardiovascular disease in aging veterans. The American Journal of Geriatric Psychiatry, 24(3), 192–200. [DOI] [PubMed] [Google Scholar]
- Boscarino JA (2006). Posttraumatic stress disorder and mortality among US Army veterans 30 years after military service. Annals of epidemiology, 16(4), 248–256. [DOI] [PubMed] [Google Scholar]
- Britvić D, Antičević V, Kaliterna M, Lušić L, Beg A, Brajević-Gizdić I, … & Pivac N (2015). Comorbidities with Posttraumatic Stress Disorder (PTSD) among combat veterans: 15 years postwar analysis. International Journal of Clinical and Health Psychology, 15(2), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochier ML, & Arwidson P (1998). Coronary heart disease risk factors in women. European heart journal, 19, A45–52. [PubMed] [Google Scholar]
- Buckley TC, Mozley SL, Bedard MA, Dewulf AC, & Greif J (2004). Preventive health behaviors, health-risk behaviors, physical morbidity, and health-related role functioning impairment in veterans with post-traumatic stress disorder. Military Medicine, 169(7), 536–540. [DOI] [PubMed] [Google Scholar]
- Buis ML (2010). Direct and indirect effects in a logit model. The Stata Journal, 10(1), 11–29. [PMC free article] [PubMed] [Google Scholar]
- Capistrant BD, Moon JR, Berkman LF, & Glymour MM (2012). Current and long-term spousal caregiving and onset of cardiovascular disease. J Epidemiol Community Health, 66(10), 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Spiro A, King L, King D, & Davison E (2009). Military Veterans. PTSD Research Quarterly, 20(3). [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, & Beckham JC (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Annals of Behavioral Medicine, 39(1), 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GH Jr, Clipp EC, Brown JS, Martin LR, & Friedman HS (2009). The lifelong mortality risks of World War II experiences. Research on Aging, 31(4), 391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler M (2011). Militarizing men: Gender, conscription, and war in post-soviet Russia. Stanford University Press. [Google Scholar]
- Finegold JA, Asaria P, & Francis DP (2013). Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. International journal of cardiology, 168(2), 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, & Rosenheck R (1999). A model of war zone stressors and posttraumatic stress disorder. Journal of Traumatic Stress: Official Publication of The International Society for Traumatic Stress Studies, 12(1), 111–126. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, & McEwen BS (2004). Posttraumatic stress disorder, allostatic load, and medical illness. In Schnurr PP & Green BL (Eds.), Trauma and health: Physical health consequences of exposure to extreme stress (p. 157–188). American Psychological Association. [Google Scholar]
- Guillemot F (2009). Death and Suffering at First Hand: Youth Shock Brigades during the Vietnam War (1950––1975). Journal of Vietnamese Studies, 4(3), 17–60. [Google Scholar]
- Hagopian A, Flaxman AD, Takaro TK, Al Shatari SAE, Rajaratnam J, Becker S, … & Murray CJ (2013). Mortality in Iraq associated with the 2003–2011 war and occupation: findings from a national cluster sample survey by the university collaborative Iraq Mortality Study. PLoS Med, 10(10), e1001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S (2008). Trajectories of functional health: the ‘long arm’ of childhood health and socioeconomic factors. Social science & medicine, 66(4), 849–861. [DOI] [PubMed] [Google Scholar]
- Hirschman C, Preston S, & Loi VM (1995). Vietnamese Casualties during the American War: A New Estimate. Population and Development Review, 21(4), 783–812. [Google Scholar]
- Jawad M, Vamos EP, Najim M, Roberts B, & Millett C (2019). Impact of armed conflict on cardiovascular disease risk: a systematic review. Heart, 105(18), 1388–1394. [DOI] [PubMed] [Google Scholar]
- Johnson SA (2017). The cost of war on public health: an exploratory method for understanding the impact of conflict on public health in Sri Lanka. PLoS One, 12(1), e0166674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Bullman TA, & Taylor JW (2006). Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Annals of epidemiology, 16(5), 381–386. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, & Mora CA (1989). Clinical evaluation of a measure to assess combat exposure. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 1(1), 53. [Google Scholar]
- Kibler JL, Joshi K, & Ma M (2009). Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behavioral Medicine, 34(4), 125–132. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Ma M, Tursich M, Malcolm L, Llabre MM, Greenbarg R, … & Beckham JC (2018). Cardiovascular risks in relation to posttraumatic stress severity among young trauma-exposed women. Journal of affective disorders, 241, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, & Samper RE (2006). Deployment Risk and Resilience Inventory: A collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology, 18(2), 89–120. [Google Scholar]
- Korinek K, Teerawichitchainan B, Zimmer Z, Brindle E, Nguyen TKC, Nguyen HM, & Tran KT (2019). Design and measurement in a study of war exposure, health, and aging: protocol for the Vietnam health and aging study. BMC public health, 19(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek K, Loebach P, & Trinh HN (2017). Military service and smoking in a cohort of northern Vietnamese older adults. International journal of public health, 62(1), 43–51. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Jones C, & Eaton WW (2009). A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychology, 28(1), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenehm S (2018). Temporal stability of risk attitudes and the impact of adverse shocks—a panel data analysis from Thailand and Vietnam. World Development, 102, 262–274. [Google Scholar]
- Matheson FI, White HL, Moineddin R, Dunn JR, & Glazier RH (2010). Neighbourhood chronic stress and gender inequalities in hypertension among Canadian adults: a multilevel analysis. Journal of Epidemiology & Community Health, 64(8), 705–713. [DOI] [PubMed] [Google Scholar]
- Miguel E, & Roland G (2011). The long-run impact of bombing Vietnam. Journal of development Economics, 96(1), 1–15. [Google Scholar]
- Moazen-Zadeh Ehsan, Khoshdel Alireza, Avakh Farhad, and Rahmani Arash. “Increased blood pressures in veterans with post traumatic stress disorder: A case-control study.” The International Journal of Psychiatry in Medicine 51, no. 6 (2016): 576–586. [DOI] [PubMed] [Google Scholar]
- Murray CJ, King G, Lopez AD, Tomijima N, & Krug EG (2002). Armed conflict as a public health problem. Bmj, 324(7333), 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole BI, & Catts SV (2008). Trauma, PTSD, and physical health: an epidemiological study of Australian Vietnam veterans. Journal of psychosomatic research, 64(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Pike D (1986). PAVN: People’s Army of Vietnam. Novato: Presidio Press. [Google Scholar]
- Poole D (2012). Indirect health consequences of war: cardiovascular disease. International journal of sociology, 42(2), 90–107. [Google Scholar]
- Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, … & Murray CJ (2015). Demographic and epidemiologic drivers of global cardiovascular mortality. New England Journal of Medicine, 372(14), 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, … & Abdollahpour I (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, & Kaplan J (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99(16), 2192–2217. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, & Spiro A (1999). Combat exposure, posttraumatic stress disorder symptoms, and health behaviors as predictors of self-reported physical health in older veterans. The Journal of nervous and mental disease, 187(6), 353–359. [DOI] [PubMed] [Google Scholar]
- Settersten RA Jr. (2006). When nations call: How wartime military service matters for the life course and aging. Research on Aging, 28, 12–36. [Google Scholar]
- Shivpuri S, Gallo LC, Crouse JR, & Allison MA (2012). The association between chronic stress type and C-reactive protein in the multi-ethnic study of atherosclerosis: does gender make a difference?. Journal of behavioral medicine, 35(1), 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai AM, Armenian HK, & Alam S (1989). Wartime determinants of arteriographically confirmed coronary artery disease in Beirut. American journal of epidemiology, 130(4), 623–631. [DOI] [PubMed] [Google Scholar]
- Smith JP, Shen Y, Strauss J, Zhe Y, & Zhao Y (2012). The effects of childhood health on adult health and SES in China. Economic development and cultural change, 61(1), 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son PT, Quang NN, Viet NL, Khai PG, Wall S, Weinehall L, … & Byass P (2012). Prevalence, awareness, treatment and control of hypertension in Vietnam—results from a national survey. Journal of human hypertension, 26(4), 268–280. [DOI] [PubMed] [Google Scholar]
- Sparén P, Vågerö D, Shestov DB, Plavinskaja S, Parfenova N, Hoptiar V, … & Galanti MR (2004). Long term mortality after severe starvation during the siege of Leningrad: prospective cohort study. bmj, 328(7430), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel P (2010). Urban refugee health: Meeting the challenges. Forced Migration Review, (34), 22. [Google Scholar]
- Stein Z, Susser M, Saenger G, & Marolla F (1975). Famine and human development: The Dutch hunger winter of 1944–1945.
- Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, … & Suglia SF (2015). Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation, 132(4), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Roberts AL, Gilsanz P, Chen Q, Winning A, … & Koenen KC (2016). Posttraumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychological medicine, 46(15), 3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MG, Ureña S, & Kail BL (2016). Service-related exposures and physical health trajectories among aging veteran men. The Gerontologist, 56(1), 92–103. [DOI] [PubMed] [Google Scholar]
- Tran BX, Moir MP, Thai TPT, Nguyen LH, Ha GH, Nguyen THT, … & Latkin CA (2018). Socioeconomic Inequalities in Health-Related Quality of Life among Patients with Cardiovascular Diseases in Vietnam. BioMed research international, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Gottschang K, & Phan TH (1998). Even the women must fight: Memories of war from North Vietnam. Wiley. [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, … & Bremner JD (2013). Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. Journal of the american college of cardiology, 62(11), 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke JM (1972). North Vietnam’s Strategy for Survival. Palo Alto: Pacific Book Publishers [Google Scholar]
- Van Minh H, Byass P, Chuc NTK, & Wall S (2006). Gender differences in prevalence and socioeconomic determinants of hypertension: findings from the WHO STEPs survey in a rural community of Vietnam. Journal of human hypertension, 20(2), 109. [DOI] [PubMed] [Google Scholar]
- von Känel R (2012). Psychosocial stress and cardiovascular risk-current opinion. Swiss Medical Weekly, 142(0304). [DOI] [PubMed] [Google Scholar]
- Wagner Z, Heft-Neal S, Bhutta ZA, Black RE, Burke M, & Bendavid E (2018). Armed conflict and child mortality in Africa: a geospatial analysis. The Lancet, 392(10150), 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajngarten M, & Silva GS (2019). Hypertension and Stroke: Update on Treatment. European Cardiology Review, 14(2), 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, … & MacLaughlin EJ (2018). ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71(19), e127–e248. [DOI] [PubMed] [Google Scholar]
- Wise PH (2017). The epidemiologic challenge to the conduct of just war: confronting indirect civilian casualties of war. Daedalus, 146(1), 139–154. [Google Scholar]
- Young Y, Korinek K, Zimmer Z, & Tran TK (unpublished results). Assessing War-related Trauma Exposure in Older Vietnamese War Survivors.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


