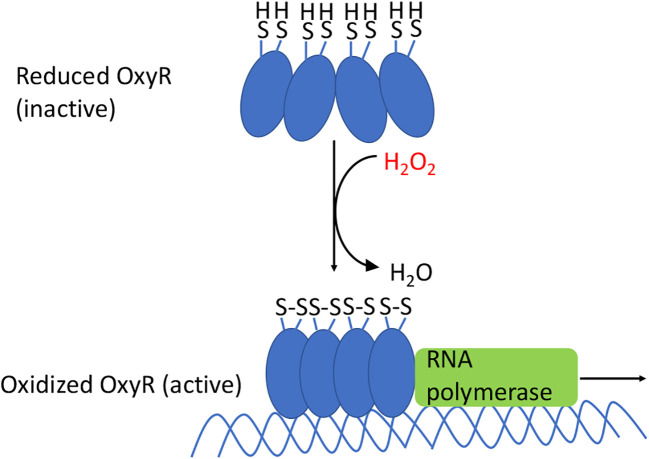
Figure 6.
OxyR activation in E. coli. The oxidation of the sensory C199 cysteine by H2O2 leads to the formation of a disulfide bond between C199 and C208. The resulting conformational change causes OxyR to bind as a tetramer to the promoter regions, which recruits RNA polymerase, and results in the transcription of genes in the OxyR regulon. In many other bacteria the reduced form also binds DNA, albeit in an elongated conformation that represses transcription; oxidation again converts it to a transcriptional activator.