Figure 9.
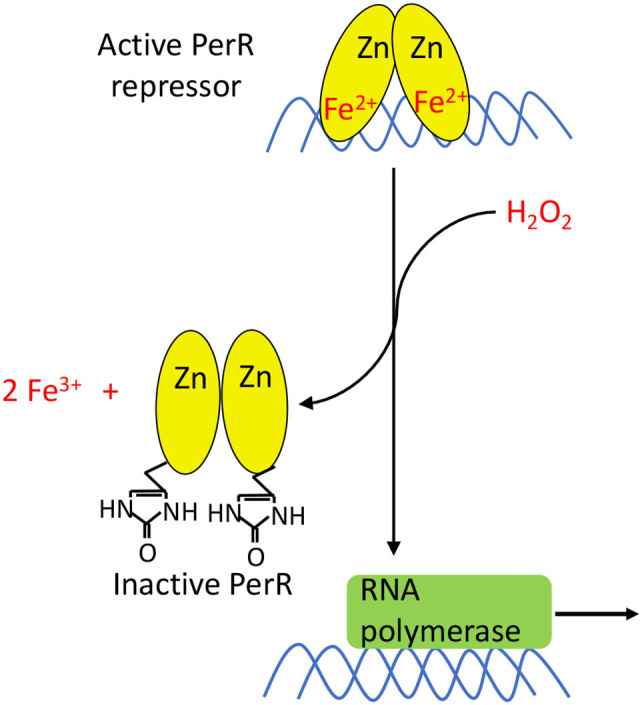
PerR activation. PerR is a dimeric DNA-binding protein, and it binds two metal ions per monomer. The first ion is a structural Zn2+ that is necessary for dimerization and structural integrity. The second metal ion enables DNA binding, and can either be Fe2+ or Mn2+. Only PerR bound to Fe2+ is responsive to H2O2. The oxidation of Fe2+ by H2O2 generates a localized hydroxyl/ferryl radical, which irreversibly oxidizes either of two His ligands (H37 or H91) to form 2-oxo-histidine. Metal binding is blocked, PerR dissociates from promoter sites, and the regulon is induced.
