Abstract
Multiple structural and functional neuroimaging measures vary over the course of the lifespan and can be used to predict chronological age. Accelerated brain aging, as quantified by deviations in the MRI-based predicted age with respect to chronological age, is associated with risk for neurodegenerative conditions, bipolar disorder, and mortality. Whether age-related changes in resting-state functional connectivity are accelerated in major depressive disorder (MDD) is unknown, and, if so, it is unclear if these changes contribute to specific cognitive weaknesses that often occur in MDD. Here, we delineated age-related functional connectivity changes in a large sample of normal control subjects and tested whether brain aging is accelerated in MDD. Furthermore, we tested whether accelerated brain aging predicts individual differences in cognitive function. We trained a support vector regression model predicting age using resting-state functional connectivity in 710 healthy adults aged 18–89. We applied this model trained on normal aging subjects to a sample of actively depressed MDD participants (n = 109). The difference between predicted brain age and chronological age was 2.11 years greater (p = 0.015) in MDD patients compared to control participants. An older MDD brain age was significantly associated with increased impulsivity and, in males, increased depressive severity. Unexpectedly, accelerated brain aging was also associated with increased placebo response in a sham-controlled trial of high-frequency repetitive transcranial magnetic stimulation targeting the dorsomedial prefrontal cortex. Our results indicate that MDD is associated with accelerated brain aging, and that accelerated aging is selectively associated with greater impulsivity and depression severity.
Subject terms: Depression, Cognitive ageing
Introduction
Normal aging is associated with declines in specific cognitive domains. For example, executive function, memory, and processing speed decline with age, whereas verbal skills, semantic knowledge, affective function, perceptual priming, and implicit learning remain relatively intact [1, 2]. Poor executive function may contribute to other age-related cognitive weaknesses, including reductions in memory performance, and visuospatial skill [3]. These age-related declines in executive function are associated with poorer quality of life [4] and an increased risk of psychiatric disorders, including in major depressive disorder (MDD [5]).
Numerous magnetic resonance imaging (MRI) studies have sought to identify functional connectivity correlates of normal brain aging and age-related executive function decline. These functional MRI (fMRI) studies of normal aging have consistently reported lower within-network resting-state functional connectivity (rsFC) in older age within executive/attentional control, default mode (DMN), and sensorimotor networks [6–8]—some of which are correlated with declines in executive function [9]. Additionally, impulsivity in healthy older adults arises from altered representations of future rewards in the prefrontal cortex [10], and deficient sensitivity to immediate rewards in the dorsal striatum [11]. However, few studies have identified predictors of increased risk for accelerated brain aging [12].
MDD may increase the risk for accelerated aging. Age-sensitive aspects of executive function, including cognitive flexibility, and attentional and motor impulsivity, are reduced in MDD [13, 14], and late-life MDD is associated with aberrant rsFC and deficits in executive function [15, 16]. Among cognitive symptoms, impulsivity may be particularly relevant to MDD outcomes. Greater impulsivity is positively correlated with depressive severity [17]. Impulsivity has shown to mediate the relationship between hopelessness and suicidality in young adult and late-life MDD [18, 19], and may contribute to the observation that aging-related executive function decline is a predictor of suicidal ideation in older adults [20]. Consequently, characterizing abnormalities of functional brain aging in MDD may provide a more complete understanding of the interplay of age-related declines in impulsivity and depressive symptomatology.
MDD is also associated with accelerated cellular and molecular aging [21, 22], and a recent structural MRI study reported accelerated age-related cortical thinning in depression [23]. Although multiple high-impact studies have characterized functional network alterations in late-life depression [15, 24], relatively few have examined connectivity changes across the lifespan. Thus, there is limited evidence that speaks directly to the question of whether or not age-related connectivity changes are accelerated in depression and, if so, how they relate to deficits in executive function and mood—a potentially critical question for understanding the biological basis of heterogeneity in depression across the lifespan.
Neuroimaging data are characterized by high interindividual variability in age-related changes [25]. This age-related variability correlates with disease risk, such as the progression of cognitive decline in Alzheimer’s disease [26], and some studies suggest that “brain age” may be a stronger risk predictor than chronological age [9, 27–31]. This hypothesis has motivated increased interest in predicting “brain age” based on neuroimaging variables and investigating whether deviations between brain age and chronological age—known as the brain-predicted age difference (brain-PAD)—are clinically relevant. To date, most brain-PAD studies have predicted age using structural MRI measures, including gray/white matter volume and cortical thickness. An older brain-PAD predicts who is more likely to progress from mild cognitive impairment to Alzheimer’s Disease [32] and is associated with increased mortality in healthy older adults [33]. Only two studies used rsFC to predict age, and both focused on healthy human subjects [34, 35]. One reported an association between brain-PAD and cognitive function in adults: brain-PADs were older in individuals with objective cognitive impairment [35]. Some [36, 37], but not all [38] brain-PAD studies using structural neuroimaging reported significantly older brain-PAD in MDD. It is unknown whether age-related changes in functional network organization are altered in depression.
Molecular markers of aging have been consistently associated with pharmacotherapy nonresponse [39–42]. Whether these age-related rsFC markers also predict poorer response to antidepressant treatments such as repetitive transcranial magnetic stimulation (rTMS) [43] has not been determined. Older chronological age was modestly correlated with rTMS nonresponse in early rTMS trials [44–48], though a recent trial using higher stimulation intensities reported that older age was associated with better response [49]. Interindividual differences in both neurophysiologic and antidepressant response to rTMS may be driven by age-related changes in rsFC. Understanding the relationship between brain aging in MDD and rTMS response may be particularly valuable as it could facilitate tailoring rTMS stimulation parameters for MDD patients.
Here, we set out to investigate whether age-related rsFC changes are accelerated in depression and to evaluate how they influence cognition, mood, and rTMS response. First, to identify patterns of brain aging based on rsFC, we trained a support vector regression model to predict chronological age using rsFC in healthy human subjects. We hypothesized that the accuracy of this model for predicting age in healthy controls would be significantly better than chance. Our second aim was to test for evidence of accelerated brain aging in depression by evaluating the accuracy of the age-prediction model in an independent sample of MDD individuals. We hypothesized that the brain-PAD would be significantly higher in MDD patients than in healthy controls. Our final aim was to test whether accelerated brain aging in MDD is associated with (1) deficits in executive function, as measured by the severity of impulsivity in the MDD group and, (2) rTMS treatment response. We hypothesized that the brain-PAD in depressed patients would correlate with greater impulsivity, indicating greater deficits in executive function. Given previous work demonstrating the relationship between cellular aging and antidepressant nonresponse, we also hypothesized that accelerated brain aging (brain-PAD) would be a negative predictor of treatment response to active rTMS targeting the dorsomedial prefrontal cortex (DMPFC), but not to placebo, in a previously published three-arm placebo-controlled clinical trial [50].
Materials and methods
Subjects
We used three neuroimaging datasets from two scanners. The first dataset came from the open-access Enhanced Nathan Kline Institute Rockland County Sample [51]. Participants between 18 and 85 years old were selected for preprocessing from Releases 1–8 of the Cross-Sectional Lifespan Connectomics Study. The second dataset included healthy older adults who were also scanned at the Nathan Kline Institute, and recruited at the Weill Cornell Medicine Institute of Geriatric Psychiatry via community advertisements for non-psychiatric comparison subjects (ClinicalTrials.gov: NCT01728194). The final dataset included actively depressed subjects and HCs, originally recruited as part of a randomized controlled trial of rTMS targeting the DMPFC ([50] ClinicalTrials.gov:NCT02702154). MDD participants were referred to the MRI-Guided rTMS Clinic at Toronto Western Hospital and HC were recruited from the community. MDD participants were randomized to receive one of two active treatments (1 Hz DMPFC-rTMS or 20 Hz DMPFC-rTMS) or placebo rTMS, twice-daily, for three weeks. In total, the three studies yielded data from 848 subjects between 18 and 89 years old (736 HC and 112 MDD; Table 1 and Fig. S2). For additional details on inclusion and exclusion criteria, comorbidities, and medication status, see the Supplementary Methods and Results. All participants provided written informed consent, and studies were approved by their respective Research Ethics Board or Institutional Review Board.
Table 1.
Gender (A) and Age (B) for all subjects.
| (A) | ||||
| NKI | TWH | WCM | MDD | |
| Male | 200 | 43 | 27 | 39 |
| Female | 373 | 54 | 39 | 72 |
| Total | 573 | 97 | 66 | 112 |
| (B) | ||||
| Site | N | Mean | SD | Min. |
| NKI | 573 | 47.62 | 18.94 | 18 |
| TWH | 97 | 36.94 | 14.67 | 18 |
| WCM | 66 | 72.42 | 6.19 | 60 |
| MDD | 112 | 38.88 | 11.76 | 18 |
| Total | 848 | 49.45 | 19.42 | 18 |
Min minimum, MDD major depressive disorder, NKI Nathan Kline Institute, TWH Toronto Western Hospital, SD standard deviation, WCM Weill Cornell Medicine.
rsfMRI data acquisition and preprocessing
Scans from 710/736 (96.47%) HC and 109/116 (93.97%) MDD participants were deemed usable by criteria defined a priori, including framewise displacement (FD), and whole-brain temporal signal-to-noise (tSNR) (Supplementary Results, Table S1 and Fig. S3). Participants’ FD was also used in all post hoc analyses to ensure that there was no association between imaging quality and predicted age associations by diagnosis or with clinical scales. We controlled for scanner-related differences in rsFC measures using ComBat Harmonization [52, 53], but to avoid biasing our held-out test sample, these controls were implemented iteratively within the model training loop and are described in the “Model training” subsection. For additional details on neuroimaging acquisition parameters and preprocessing procedures, see the Supplementary Methods.
Clinical assessments
At baseline, MDD participants completed the 17-item Hamilton Depression Rating Scale (HDRS [54]), and the Barratt Impulsiveness Scale-11 (BIS-11 [55]). The BIS-11 is a 30-item self-reported questionnaire assessing attentional, motor, and non-planning impulsivity. Percent HDRS improvement from baseline to 1-month post-treatment was also collected.
Model training
All models were implemented using LIBSVM V3.23 [56] in Matlab V2019a (The MathWorks, Inc., Natick, Massachusetts, USA), using an ε-support vector regression with a radial basis function. To establish model performance and optimize three parameters (number of rsFC features, cost, and gamma parameters), 1000 iterations of the following were performed. First, whole-brain correlation matrices representing 33,411 rsFC features (using regions of interest visualized in Fig. S1) were partitioned into training (90%) and test sets (10%). Next, we conducted parametric ComBat Harmonization [52, 53] on the training set, with age as a covariate, to account for scanning acquisition differences. The resultant parametric adjustments derived from the training set were applied to the test set to account for scanner differences.
Next, we ranked rsFC features that were significantly and stably correlated with age. To generate these rankings, we performed bootstrapping with replacement (5000 iterations) on the training set, parametrically correlating age with all 33,411 rsFC features. Features were considered stable if they were correlated with age at a threshold of p < 0.0001 across >80% of the bootstrapping iterations. Stable features were ranked by the mean p value for their correlation with age across the 5000 bootstrapping iterations.
We then performed a support vector regression with a grid search to optimize the number of features (50–600 features), the cost parameter (10e−5 to 10e5), and gamma parameter (10e−5 to 10e−2). This resulted in 528 models per iteration; each model was applied to the held-out test set and the squared correlation coefficient was extracted for each combination of model parameters. The mean squared correlation coefficient across all iterations was used to determine the optimal model parameters.
In order to test whether the optimal combination of model parameters produced a significantly predictive model, we then repeated the aforementioned 1000 training and testing iterations, shuffling the ages of individuals at each iteration. If there were fewer than 600 stably predictive rsFC features during feature selection, rsFC features were ranked on the mean p values generated using all 5000 bootstraps irrespective of subsample stability. The null distribution generated by this analysis was used to evaluate the statistical significance of our model’s performance.
Performance in MDD
Once the optimal hyperparameters were identified, feature z-normalization, feature selection, and modeling were repeated using leave-one-out cross-validation to generate HC brain-PAD scores to compare against MDD brain-PAD. Brain-PAD was calculated as the predicted age minus chronological age. A final model was generated using the complete HC set; this model was applied to the MDD dataset, and we calculated the squared correlation coefficient and brain-PAD. To describe the relationship between age and rsFC features by diagnosis, we also repeated bootstrapping with replacement with the MDD group. Differences in rsFC-age relationships between MDD and controls were assessed by correlating brain-age associations by ROI between the MDD and controls; significantly negative correlations indicate a difference in the whole-brain rsFC-age associations for a given ROI between the MDD and HC groups. To test whether predicted brain age in MDD patients was larger than in HC, we used a univariate generalized linear model (GLM) with age, gender, and diagnosis as main effects to compare MDD brain-PAD against the brain-PAD from HC from the same site and scanner. We also tested whether brain-PAD of both HC and MDD differed by gender or correlated with neuroimaging quality measures (FD and whole-brain signal-to-noise).
Finally, we tested whether MDD brain-PAD was associated with baseline HDRS severity, executive function, and symptom improvement. Due to recent debate on the factor structure of the BIS-11 [57], we first performed an exploratory factor analysis on the BIS-11 items, using maximum likelihood extraction with a varimax rotation. Eigenvalues generated from a parallel analysis was used to identify the optimal number of factors. Next, we performed a GLM predicting brain-PAD, with gender as a factor, and age, BIS-11 factors, and HDRS baseline score as covariates. Main effects for all variables and two-way interactions only for age, gender, and HDRS score were included in the GLM. Second, to test whether brain-PAD was associated with individual differences in rTMS response, we conducted a GLM with percent HDRS improvement as the dependent variable and assessed whether there was a main effect of brain-PAD, age, gender, or treatment arm on percent HDRS improvement. We also modeled brain-PAD*Group (1 Hz versus 20 Hz; active versus placebo), Age* brain-PAD*Group and Gender* brain-PAD*Group interactions to determine whether there were any interactions between brain-PAD and antidepressant response by treatment arm. We accounted for multiple comparisons across the three GLMs using a Bonferroni correction.
Results
Shared age-related rsFC changes in HC and MDD
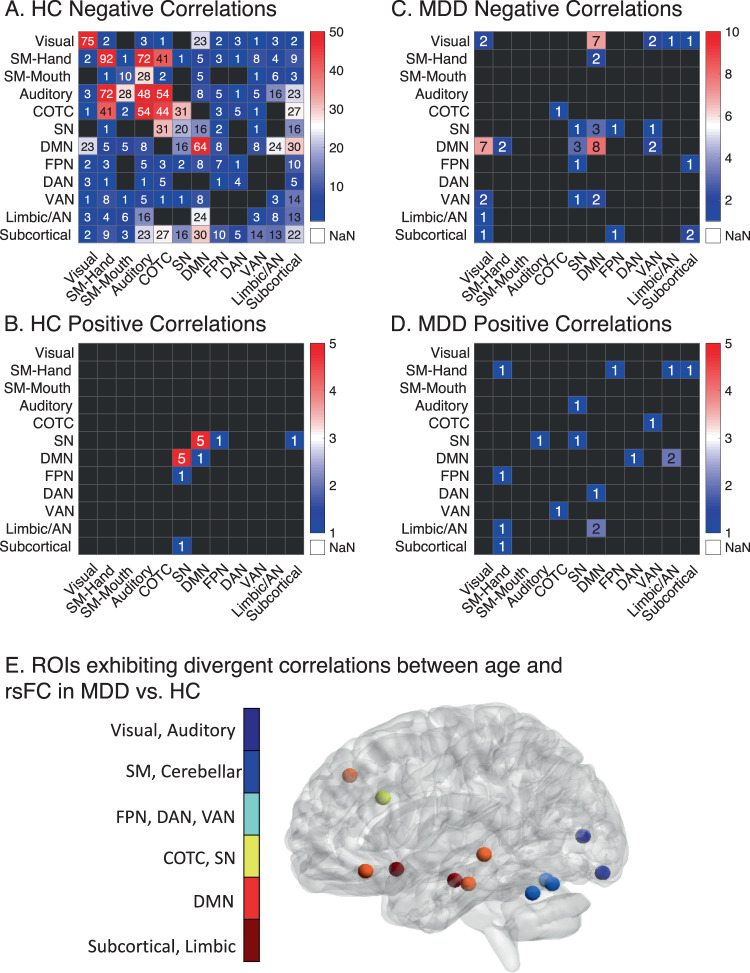
In order to characterize age-related rsFC changes and test whether similar changes occur in HC and MDD, we constructed whole-brain correlation matrices depicting the association between age and rsFC and identified both shared and divergent patterns of rsFC in the two samples. In HC, 962 out of 33,411 rsFC features were deemed stably predictive with age. rsFC decreased with chronological age in 954 of these rsFC features (mean correlation = −0.22 ± 0.04, range = −0.48 to −0.18), especially those involving within-network connectivity in sensorimotor, auditory, and cingulo-opercular task control networks and within a limbic network, which included the bilateral hippocampi, amygdalae, and subgenual cingulate (Fig. 1A). rsFC increased with chronological age in just 8 of 962 rsFC features (mean correlation = 0.20 ± 0.02, range = 0.18–0.22) involving connectivity predominately between just two networks: posterior DMN nodes and dorsal anterior cingulate node of the salience network (Figs. 1B and S4). MDD participants had similar trend of decreased age-related network associations in rsFC (Fig. 1C, D). However, 13 of 259 ROIs exhibited age-related rsFC changes that significantly differed between HC and MDD. These ROIs were predominantly within the DMN and limbic networks, and included the bilateral subgenual cingulate, and left hippocampus, lateral orbitofrontal, and dorsolateral prefrontal cortex (Fig. 1E, Supplementary Results).
Fig. 1. Chronological age is associated with decreased within-network rsFC and increased between-network rsFC between the SN and DMN in both HC and MDD.
The counts of positive (A) and negative (B) correlation coefficients that met stably predicted the relationship between age and rsFC in HC (p < 0.0001, 80% of bootstrapping iterations, 5000 iterations). Each cell represents the number of within- or between-network features that stably correlated with chronological age in HC. The counts of positive (C) and negative (D) correlation coefficients that met stably predicted the relationship between age and rsFC in MDD. As a priori-defined stability criteria failed to yield any rsFC features, we opted for a more liberal height and stability threshold for this sample (p < 0.0005, 66.7% of bootstrapping iterations, 5000 iterations). E Regions of interest where its whole-brain rsFC correlations with age significantly differed between HC and MDD (rho range = −0.29 to −0.15, FDR-p < 0.04). Briefly, most of the differences between HC and MDD in rsFC correlations with age involved default mode network and subgenual cingulate rsFC. For an additional description of these differences, see the Supplementary Results. AN: affective network, COTC: cingulo-opercular task control network, DAN: dorsal attention network, DMN: default mode network, FPN: frontoparietal network, SM: sensorimotor, SN: salience network, VAN: ventral attention network.
Robust rsFC-based prediction of chronological age in HC
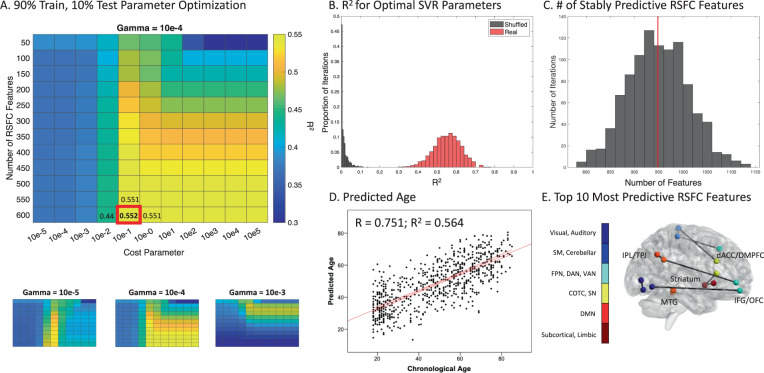
Having identified correlations between rsFC and chronological age in healthy controls, we trained a support vector regression model to predict chronological age based on the most stable age-predictive rsFC features. To identify the optimal hyperparameters, we implemented a grid search to tune the number of rsFC features, and the cost and gamma parameters, optimizing for the explained variance between the predicted and chronological age in a held-out HC sample. The best performing parameters were 600 rsFC inputs, a cost parameter of 0.1, and a gamma parameter of 0.0001 (Fig. 2A). Performance of the model trained on the real data was superior to that of models trained on shuffled data (Fig. 2B; p < 0.001). The mean R2 for the same parameters in the shuffled data was 0.014 ± 0.019. Across all training iterations, 948 ± 62 rsFC features were stably correlated with chronological age (Fig. 2C). In contrast, there were 29 ± 24 stable features over all iterations using shuffled data.
Fig. 2. Tuning and training a support vector regression model predicting brain age based on rsFC in HC.
A Grid search for optimizing SVR model parameters. Heatmaps of R2 representing the relationship between the chronological and predicted age in held-out data as a function of gamma and cost parameters, and number of rsFC features. The best combination of parameters is enlarged and presented in the red box (mean R2 = 0.552 ± 0.071). B Histogram visualizing the R2 across all 1000 training iterations for the best combination of parameters in real (red) and shuffled (gray) data. Real data R2 95% confidence interval [CI]: 0.403 0.682, range: 0.312–0.764; shuffled data R2 CI: 0.000–0.071, range: 1.129e − 9 to 0.136. C Histogram representing the number of rsFC features stably predictive of age across all 1000 training iterations. The vertical red line indicates the mean. D Scatterplot representing the correlation between chronological and predicted age in HC. The red lines indicate the regression line and the 95% confidence interval. E The top 10 most predictive rsFC features ranked by support vector regression weights. These features consisted of rsFC between: dorsal anterior cingulate cortex/DMPFC and motor regions; bilateral dorsal and ventral striatum; striatum and right inferior frontal gyrus; right IFG and orbitofrontal cortex; right parietal and visual regions; and between the bilateral medial temporal gyrus. dACC: dorsal anterior cingulate cortex, DMPFC: dorsomedial prefrontal cortex, IFG: inferior frontal gyrus, IPL: inferior parietal lobule, MTG: medial temporal gyrus, OFC: orbitofrontal cortex, TPJ: temporoparietal junction.
In preparation for comparing the brain-PAD of HC and MDD subjects, we performed two analyses to estimate brain-PAD (prediction error) in HC in held-out data using either leave-one-out cross-validation or ten-fold cross-validation. In leave-one-out cross-validation, the mean brain-PAD was 0.03 ± standard deviation 12.90 years (absolute brain-PAD = 10.50 ± 7.48 years; Fig. S5), and the correlation coefficient between the predicted and chronological age was 0.75 (Fig. 2D). A single ten-fold cross-validation using the entire sample or only the Rockland sample alone yielded similar results. For the entire dataset, the mean brain-PAD was 0.02 ± 13.25 years (absolute brain-PAD = 10.80 ± 7.66 years), and the correlation coefficient between the predicted and chronological age was 0.73. For the SVR using only the Rockland dataset, the brain-PAD was −0.15 ± 12.92 years (absolute brain-PAD = 10.58 ± 7.41, correlation coefficient = 0.73). Brain-PAD was not associated with demographic or fMRI quality control variables (Supplementary Results). To quantify the impact of scanner and acquisition parameter heterogeneity on SVR performance, we performed two supplemental analyses, training our model on two sites and testing on the third, with and without ComBat Harmonization for scanner-related differences. As expected, SVR performance was lower in both models (r = 0.55 and r = 0.28, respectively) but remained significantly predictive (p = 0.001–0.014) (Supplementary Results). The ten most predictive rsFC features were identified using the top 10 weights in the support vector regression model (Fig. 2E).
Functional brain aging is accelerated in MDD
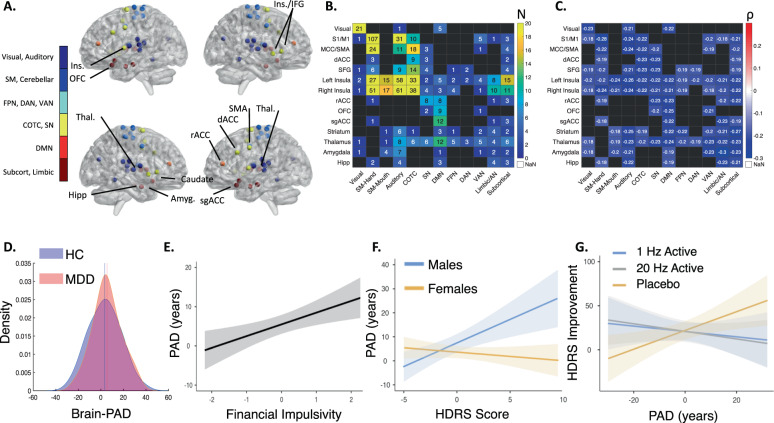
In order to determine whether brain aging was accelerated in MDD patients relative to HC, a final model was trained using the entire HC dataset and optimal hyperparameters. The 600 rsFC features used in this model (Fig. 3A–C) consisted predominantly of decreased intra-network rsFC correlating with chronological age, especially within the sensorimotor, visual, auditory, salience, and cingulo-opercular task control networks.
Fig. 3. Input rsFC features for the final support vector regression model and brain-PAD associations in MDD.
A Top 40 regions of interest predicting age, colored by brain network. The top ROIs were identified by ranking ROIs by the number of rsFC input features included in the model. B The number of rsFC features included in the model by within- or between-network rsFC of the top 40 ROIs. C The mean rsFC correlation with chronological age for within- and between-network rsFC of the top 40 ROIs. D Kernel density estimations of the brain-PAD in the MDD and HC sample from the same site and scanner. Brain-PAD in MDD was significantly higher than that of HC from the same site and scanner (Estimate = −3.66, SE = 1.49, p = 0.015, HC = 3.38 ± 14.06 years; MDD = 5.49 ± 12.65 years). E Greater financial impulsivity on the BIS-11 factor was associated with an older brain-PAD (Estimate = 3.08, SE = 1.00, p = 0.003); factor scores are normalized and account for other regressors included in the GLM. F Interaction of HDRS score and gender; males with high depressive symptomatology had older brain-PADs (Estimate = −2.27, SE = 0.69, p = 0.002); HDRS scores are normalized and account for other regressors included in the GLM. G HDRS percent improvement in the placebo-controlled TMS trial (PAD*Group (Placebo > Active) Estimate = 1.43, SE = 0.49, p = 0.004). Improvements only in the placebo arm were significantly associated with brain-PAD (Placebo estimate = 0.91, SE = 0.35, p = 0.015; 20 Hz Estimate = −0.40, SE = 0.71, p = 0.58; 1 Hz Estimate = −0.26, SE = 0.47, p = 0.59). Amyg: amygdala, AN: affective network, COTC: cingulo-opercular task control network, dACC: dorsal anterior cingulate cortex, DAN: dorsal attention network, DMN: default mode network, FPN: frontoparietal network, HC: healthy controls, HDRS: Hamilton Depression Rating Scale, Hipp hippocampus, IFG: inferior frontal gyrus, Ins insula, MDD: major depressive disorder, OFC: orbitofrontal cortex, rACC: rostral anterior cingulate cortex, PAD: brain-predicted age difference, SM: sensorimotor network, sgACC: subgenual anterior cingulate cortex, SMA supplementary motor area, SN: salience network, Thal thalamus, VAN: ventral attention network.
This model was then applied to a separate set of rsFC data from MDD patients, and we compared brain-PAD in the MDD sample with the leave-one-out cross validated HC brain-PAD. To avoid scanner-related confounds, we restricted this comparison to HC subjects scanned on an identical pulse sequence on the same scanner as our MDD sample. The MDD brain-PAD was 2.11 years higher than that of HC collected from the same site and scanner (HC = 3.38 ± 14.06 years; MDD = 5.49 ± 12.65 years). To compare the predicted age difference in MDD subjects relative to HC, we implemented a GLM testing for effects of diagnosis, age, and gender (Table 2A; full model R2 = 0.42). There were significant main effects for age and diagnosis (such that brain-PAD was significantly higher in MDD patients; Fig. 3D), but not for gender. Together, these results indicate functional brain aging is accelerated in depression compared to HCs, as indexed by increased brain-PAD in the MDD sample.
Table 2.
Parameter estimates for all main effects and significant interactions.
| Estimate | SE | 95% confidence interval | z | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| (A) | ||||||
| Gender | −0.74 | 1.49 | 0.03 | 8.85 | −0.50 | 0.619 |
| Diagnosis | −3.66 | 1.49 | 0.001 | 0.48 | −2.45 | 0.015 |
| Age | −0.66 | 0.06 | 0.46 | 0.58 | −11.42 | <0.001 |
| (B) | ||||||
| Age | −0.58 | 0.10 | −0.78 | −0.37 | −5.51 | <0.001 |
| Motor/non-planning impulsivity | 0.74 | 0.99 | −1.21 | 2.68 | 0.74 | 0.461 |
| Motor impulsivity | −0.70 | 1.02 | −2.70 | 1.30 | −0.69 | 0.496 |
| Attentional impulsivity | 1.75 | 1.11 | −0.44 | 3.93 | 1.57 | 0.122 |
| Deficits in problem solving | −0.21 | 1.03 | −2.23 | 1.80 | −0.21 | 0.835 |
| Financial impulsivity | 3.08 | 1.00 | 1.11 | 5.05 | 3.07 | 0.003 |
| HDRS | 0.79 | 0.33 | 0.14 | 1.43 | 2.39 | 0.019 |
| Gender | −3.72 | 2.21 | −8.04 | 0.60 | −1.69 | 0.096 |
| HDRS score*Gender | −2.27 | 0.69 | −3.62 | −0.92 | −3.29 | 0.002 |
| (C) | ||||||
| Gender | 9.39 | 5.96 | −2.29 | 21.06 | 1.58 | 0.119 |
| Age | 0.36 | 0.30 | −0.24 | 0.95 | 1.17 | 0.244 |
| PAD | 0.11 | 0.28 | −0.44 | 0.66 | 0.40 | 0.693 |
| Group (1 Hz > 20 Hz) | 0.08 | 7.75 | −15.11 | 15.27 | 0.01 | 0.992 |
| Group (Placebo > Active) | 1.20 | 6.64 | −11.81 | 14.22 | 0.18 | 0.857 |
| PAD*Group (1 Hz >20 Hz) | −0.13 | 0.56 | −1.23 | 0.98 | −0.22 | 0.824 |
| PAD*Group (Placebo > Active) | 1.43 | 0.49 | 0.48 | 2.39 | 2.94 | 0.004 |
(A) GLM assessing brain-PAD in MDD and HC. (B) GLM assessing pretreatment clinical measures (BIS-11 factors and HDRS) and brain-PAD in the MDD group. (C) GLM assessing the relationship between brain-PAD and TMS response. Bold and underlined uncorrected p values are significant after multiple comparisons correction. All parameter estimates are summarized in Supplementary Table S3.
HDRS Hamilton Rating Scale for Depression, PAD brain-predicted age difference score.
Accelerated brain aging predicts financial impulsivity and depression severity in men
Previous studies indicate that accelerated aging is associated with increased clinical symptomatology and deficits in executive function. To this end, we tested whether MDD brain-PAD was associated with HDRS severity, and cognitive function as indexed by the BIS-11. To reduce the dimensionality of the BIS-11 data [57], we first performed an exploratory factor analysis on the BIS-11 items. The exploratory factor analysis revealed five BIS-11 factors (Table S2). These five factors were: (1) low motor and non-planning impulsivity, (2) high motor impulsivity, (3) attentional impulsivity and restlessness, (4) deficits in problem solving and puzzles, and (5) financial impulsivity. We found that brain-PAD was strongly associated with individual differences in the financial impulsivity factor, but not the other four factors (Fig. 3E and Table 2B, model R2 = 0.60). Higher financial impulsivity was associated with higher brain-PAD; this factor was associated with the BIS-11 items related to saving, spending, and having extraneous thoughts (Table S2). There was also a significant interaction between gender and HDRS score, such that depressed men with greater depression severity had an increased brain-PAD (Fig. 3F).
Previous studies indicate biological markers of aging such as telomere length are associated with pharmacotherapy nonresponse [39–42] and chronological age is modestly correlated with rTMS nonresponse [49, 58], leading us to hypothesize accelerated brain aging as indexed by the brain-PAD would be associated with rTMS nonresponse. Thus, we tested whether pretreatment brain-PAD predicted improvements to active or placebo DMPFC-rTMS in a recently published, sham-controlled trial (Table 2C; model R2 = 0.20). Unexpectedly, we found brain-PAD significantly predicted treatment response in the placebo group, but not in either active rTMS arm (Fig. 3F). The two three-way interactions were not significant, meaning neither residual age nor gender effects influenced the relationship between treatment arm, brain-PAD, and HDRS improvement. FD did not impact the results of any of the three GLMs (Supplementary Results).
Discussion
The principal finding is that brain aging was accelerated in MDD patients. We identify robust age-related correlations in specific brain networks in a large-scale multisite sample. Of note, there is considerable overlap between networks exhibiting age-related changes and those that are altered in depression, indicating that they could contribute significantly to heterogeneity in depression pathophysiology across the lifespan. On average, brain-PAD was modestly but significantly elevated in MDD patients compared to HC. Interestingly, in depressed men but not in depressed women, more severe depressive symptoms were associated with older brain-PADs. Furthermore, brain-PAD was positively associated with increased financial impulsivity. Unexpectedly, brain-PAD was not associated with rTMS response but was positively correlated with improvement in the placebo arm.
Brain-PAD was 2.11 years higher in MDD subjects compared to HC. Some [36, 37], but not all [38] brain-PAD studies using structural neuroimaging reported significantly older brain-PAD in MDD samples. Consistent with our findings, a large, recent study focused on structural measures [59] showed this effect is small, which might explain this inconsistency in previous studies. It is also worth noting that our model included rsFC from nodes commonly associated with depression pathophysiology—including the subgenual cingulate, hippocampus, insula, and amygdala [60]—and depression-related abnormalities in rsFC in these regions may contribute to a significantly older brain-PAD in MDD. Combining the two neuroimaging modalities is a way to bolster prediction accuracy [35]. A multimodal model is therefore a logical next step to gain a more comprehensive understanding of brain aging in MDD.
We found impulsivity specifically in economic decision-making and extraneous thoughts significantly correlated with brain-PAD in the MDD sample. It should be noted that the specificity of this finding was not predicted a priori, and our data do not rule out associations with other aspects of impulsivity. Although normal aging correlates with deficits in complex financial decision-making, simple financial decision-making, such as paying bills, remains intact [61]. Furthermore, other aspects of motor and non-planning impulsivity, such as risk-taking and sensation-seeking, are negatively correlated with age [62]. Previous studies have shown that decision-making changes during aging are associated with activity in the ventral striatum during monetary loss anticipation [63] and altered PCC rsFC [64]. Interestingly, impulsivity during economic decision-making is also associated with rsFC within the frontoparietal and cingulo-opercular networks that were heavily represented in our model [65], and in other RSFC models predicting interindividual differences in delay discounting [66].
It is also worth noting that employment stability or socioeconomic status may moderate the relationship between increased financial impulsivity and brain age in MDD. The MDD group in this study were treatment-resistant; this population is more likely to be unemployed and have a significantly lower socioeconomic status relative to nontreatment-resistant MDD or healthy individuals [67]. Socioeconomic status is significantly correlated with maladaptive economic decision-making [68] and worsening brain aging markers like network modularity [69] and hippocampal volume [70]. A logical next step would be to address the independent contributions of such social inequities in the complex interplay between the neurophysiological correlates of aging and psychiatric disorders.
We also found that accelerated brain aging correlated with more severe depressive symptoms in depressed men, but not in depressed women. Previously published studies report larger brain-PADs among nondepressed males [33] and robust sex differences in molecular aging factors, including genomic stability and epigenetic methylation [71], and rsFC [72]. However, gender did not otherwise account for differences in brain-PAD in MDD or HC in our analysis. Interestingly, this finding was not replicated in a recent study investigating structural brain aging in MDD; there was no association between brain age and clinical factors like severity or age of onset [59]. While we did not formally collect age of depression episode onset, future studies should aim to address the interplay between clinical or demographic factors in MDD and brain aging. Further studies will be needed to determine whether there is a causative link between depression severity and brain aging and whether these mechanisms are modulated by sex.
Contrary to our prediction, brain-PAD was not significantly correlated with the antidepressant response to active DMPFC-rTMS. rTMS was delivered at 120% of the resting motor threshold, possibly minimizing the negative impact of any age-related atrophy, increased scalp-to-cortex distance [73], or associated disruptions in rsFC. Furthermore, previously reported studies reported that cortico-striato-thalamocortical and frontolimbic circuitry are implicated in DMPFC-rTMS response [74–76]. These rsFC features were less predominant in our model predicting age, possibly rendering it a poor predictor of active DMPFC-rTMS response. Surprisingly, we found a strong positive correlation between brain-PAD and placebo rTMS response. Previous studies indicate either no correlation or a modest anticorrelation between placebo responsivity and chronological age [77]. However, increased RSFC between the DMN and SN has been observed as a predictor of antidepressant placebo response [78]. Interestingly, all positively correlated RSFC features with age involved the SN and/or DMN, five of which were inter-network RSFC between these two networks.
Several limitations should be noted. First, like several studies on this topic [36–38, 59], we tested for evidence of accelerated brain aging in MDD by training a model to predict healthy aging and applying it to MDD patients. This approach is advantageous because it provides a means of identifying rsFC patterns that are characteristic of healthy aging and then testing whether they are altered in MDD. As in prior studies [36–38, 59], we interpret this finding of increased brain-PAD in MDD as evidence of accelerated or potentially pathological brain aging, but other factors may also contribute to an increased prediction error in MDD. For example, to the extent that age-related changes in rsFC in HC are largely non-overlapping with age-related changes occurring in MDD subjects, this could also contribute to an increased prediction error. Arguing against this interpretation, the data in Fig. 1 indicate that age-related rsFC changes are similar in both HC and MDD subjects. It is also noteworthy that the absolute brain-PAD in HC was slightly higher than in previously reported studies predicting age using structural MRI [36, 59, 79–83]. This study was also not sufficiently powered to train separately for males and females; brain-PAD may differ between males and females in separately trained models [33]. However, there were no main effects of gender on brain-PAD, and gender was accounted for in each GLM. There were also significant site differences, both in terms of subjects’ age and rsFC quality. Ideally, all subjects should be acquired with the same scanner parameters at the same site to minimize fMRI-related confounds. To account for this potential issue, we corrected for site differences in the training and test sets of every model. Additionally, brain-PAD was not associated with rsFC data quality in either HC or MDD. We also compared brain-PAD in MDD and HC participants with neuroimaging data acquired from the same scanner to mitigate any effects related to age, inclusion criteria, scanner acquisition parameters, or data quality differences across scanners. Lastly, it is unclear whether increased brain-PAD is driving increased impulsivity and depressive symptom severity or vice versa. Further studies will be needed to elucidate the uni- and bi-directional relationships between age and behavior.
In conclusion, this study predicted brain age in HC and MDD patients using rsFC. Functional brain aging was accelerated in MDD patients compared to HC, and accelerated brain aging in MDD was associated with increased impulsivity. The study provides evidence for the notion that MDD is associated with accelerated brain aging, and that accelerated aging is associated with worsened impulsivity and depression severity. Establishing these biologically based relationships will be critical to more comprehensively understand the etiology and heterogeneity of MDD, with the hopes of identifying novel treatments to address the significant personal and economic burden of this disorder.
Funding and disclosure
KD is supported by a Canadian Institutes for Health Research Banting Postdoctoral Fellowship and has received a Canadian Institutes for Health Research Vanier Scholarship. LWV has received grants from the NIMH. JD has received research support from the Arrell Family Foundation, the Buchan Family Foundation, Brain Canada, the Canadian Biomarker Integration Network in Depression, the Canadian Institutes of Health Research (CIHR), the Klarman Family Foundation, NIH, the Ontario Brain Institute, the Toronto General and Western Hospital Foundation, and the Weston Family Foundation; he has received travel stipends from Lundbeck and ANT Neuro; he has served as an advisor for BrainCheck, Restorative Brain Clinics, NeuroStim Clinics, and TMS Neuro Solutions. FMG and CL are supported by grants from the National Institute of Mental Health, the National Institute on Drug Abuse, the Rita Allen Foundation, the Klingenstein-Simons Foundation, the Brain and Behavior Research Foundation, the Hope for Depression Research Foundation, and the Pritzker Neuropsychiatric Disorders Research Consortium. CL is listed as an inventor on Cornell University patent applications on neuroimaging biomarkers for depression that are pending or in preparation. The authors report no other financial relationships or conflicts.
Supplementary information
Author contributions
KD: study concept, data collection, data curation, formal analysis, writing (original draft), writing (reviewing, editing, and approval); LWV: study concept, data collection, data curation, formal analysis, writing (reviewing, editing, and approval); JD: data collection, writing (reviewing, editing, and approval); FMG: study concept, writing (reviewing, editing, and approval); CL: study concept, writing (reviewing, editing, and approval).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Katharine Dunlop, Email: kad2032@med.cornell.edu.
Faith M. Gunning, Email: fgd2002@med.cornell.edu
Conor Liston, Email: col2004@med.cornell.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-00967-x.
References
- 1.Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 2.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–60. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–9. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 4.Lara E, Koyanagi A, Caballero F, Domènech-Abella J, Miret M, Olaya B, et al. Cognitive reserve is associated with quality of life: a population-based study. Exp Gerontol. 2017;87:67–73. doi: 10.1016/j.exger.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A, Deaton A, Stone AA. Subjective wellbeing, health, and ageing. Lancet. 2015;385:640–8. doi: 10.1016/S0140-6736(13)61489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24:49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. Neuroimage. 2016;133:321–30. doi: 10.1016/j.neuroimage.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24:2186–98. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- 10.Eppinger B, Heekeren HR, Li S-C. Age-related prefrontal impairments implicate deficient prediction of future reward in older adults. Neurobiol Aging. 2015;36:2380–90. doi: 10.1016/j.neurobiolaging.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS ONE. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 2017;160:32–40. doi: 10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- 13.Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140:113–24. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Fortgang RG, Hultman CM, van Erp TGM, Cannon TD. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: testing for shared endophenotypes. Psychol Med. 2016;46:1497–507. doi: 10.1017/S0033291716000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Respino M, Hoptman MJ, Victoria LW, Alexopoulos GS, Solomonov N, Stein AT, et al. Cognitive control network homogeneity and executive functions in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:213–21. doi: 10.1016/j.bpsc.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandelman JA, Albert K, Boyd BD, Park JW, Riddle M, Woodward ND, et al. Intrinsic functional network connectivity is associated with clinical symptoms and cognition in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:160–70. doi: 10.1016/j.bpsc.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubczyk A, Klimkiewicz A, Topolewska-Wochowska A, Serafin P, Sadowska-Mazuryk J, Pupek-Pyzioł J, et al. Relationships of impulsiveness and depressive symptoms in alcohol dependence. J Affect Disord. 2012;136:841–7. doi: 10.1016/j.jad.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Jiang N, Cheung EFC, Sun H, Chan RCK. Role of depression severity and impulsivity in the relationship between hopelessness and suicidal ideation in patients with major depressive disorder. J Affect Disord. 2015;183:83–9. doi: 10.1016/j.jad.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld E, O’Rourke N. Impulsivity and hopelessness as predictors of suicide-related ideation among older adults. Can J Psychiatry. 2009;54:684–92. doi: 10.1177/070674370905401005. [DOI] [PubMed] [Google Scholar]
- 20.Rosowsky E. Suicidal behavior in the nursing home and a postsuicide intervention. Am J Psychother. 1993;47:127–42. doi: 10.1176/appi.psychotherapy.1993.47.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–38. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- 22.Han LKM, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175:774–82. doi: 10.1176/appi.ajp.2018.17060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eavani H, Habes M, Satterthwaite TD, An Y, Hsieh M-K, Honnorat N, et al. Heterogeneity of structural and functional imaging patterns of advanced brain aging revealed via machine learning methods. Neurobiol Aging. 2018;71:41–50. doi: 10.1016/j.neurobiolaging.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moradi E, Pepe A, Gaser C, Huttunen H, Tohka J, Alzheimer’s Disease Neuroimaging Initiative. Machine learning framework for early MRI-based Alzheimer’s conversion prediction in MCI subjects. Neuroimage. 2015;104:398–412. doi: 10.1016/j.neuroimage.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson J, Pudas S, Nilsson L-G, Nyberg L. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging. 2014;35:2107–17. doi: 10.1016/j.neurobiolaging.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Fjell AM, Sneve MH, Storsve AB, Grydeland H, Yendiki A, Walhovd KB. Brain events underlying episodic memory changes in aging: a longitudinal investigation of structural and functional connectivity. Cereb Cortex. 2016;26:1272–86. doi: 10.1093/cercor/bhv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard C, Dilharreguy B, Helmer C, Chanraud S, Amieva H, Dartigues J-F, et al. PCC characteristics at rest in 10-year memory decliners. Neurobiol Aging. 2015;36:2812–20. doi: 10.1016/j.neurobiolaging.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Fjell AM, Sneve MH, Grydeland H, Storsve AB, Walhovd KB. The disconnected brain and executive function decline in aging. Cereb Cortex. 2017;27:2303–17. doi: 10.1093/cercor/bhw082. [DOI] [PubMed] [Google Scholar]
- 31.Baker GT, Sprott RL. Biomarkers of aging. Exp Gerontol. 1988;23:223–39. doi: 10.1016/0531-5565(88)90025-3. [DOI] [PubMed] [Google Scholar]
- 32.Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H, Alzheimer’s Disease Neuroimaging Initiative. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS ONE. 2013;8:e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle N, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23:1385–92. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Satterthwaite TD, Fan Y. Brain age prediction based on resting-state functional connectivity patterns using convolutional neural networks. Proc IEEE Int Symp Biomed Imaging. 2018;2018:101–4. doi: 10.1109/ISBI.2018.8363532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–88. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40:1140–53. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolenic M, Franke K, Hlinka J, Matejka M, Capkova J, Pausova Z, et al. Obesity, dyslipidemia and brain age in first-episode psychosis. J Psychiatr Res. 2018;99:151–8. doi: 10.1016/j.jpsychires.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann T, van der Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019;22:1617–23. doi: 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–54. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–72. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasgon N, Lin KW, Lin J, Epel E, Blackburn E. Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: a preliminary study. Transl Psychiatry. 2016;6:e709. doi: 10.1038/tp.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, Lindqvist D, et al. Leukocyte telomere length predicts SSRI response in major depressive disorder: a preliminary report. Mol Neuropsychiatry. 2016;2:88–96. doi: 10.1159/000446500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 44.Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci. 1998;10:20–5. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 45.Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9:641–54. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 46.Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S, Robinson RG. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. 2001;13:225–31. doi: 10.1017/s1041610201007608. [DOI] [PubMed] [Google Scholar]
- 47.Mosimann UP, Marré SC, Werlen S, Schmitt W, Hess CW, Fisch HU, et al. Antidepressant effects of repetitive transcranial magnetic stimulation in the elderly: correlation between effect size and coil-cortex distance. Arch Gen Psychiatry. 2002;59:560–1. doi: 10.1001/archpsyc.59.6.560. [DOI] [PubMed] [Google Scholar]
- 48.Mosimann UP, Schmitt W, Greenberg BD, Kosel M, Müri RM, Berkhoff M, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126:123–33. doi: 10.1016/j.psychres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, et al. Trajectories of response to dorsolateral prefrontal rTMS in major depression: a THREE-D study. Am J Psychiatry. 2019;176:367–75. doi: 10.1176/appi.ajp.2018.18091096. [DOI] [PubMed] [Google Scholar]
- 50.Dunlop K, Sheen J, Schulze L, Fettes P, Mansouri F, Feffer K, et al. Dorsomedial prefrontal cortex repetitive transcranial magnetic stimulation for treatment-refractory major depressive disorder: a three-arm, blinded, randomized controlled trial. Brain Stimul. 2019. 10.1016/j.brs.2019.10.020. [DOI] [PubMed]
- 51.Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, et al. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front Neurosci. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 53.Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70. doi: 10.1016/j.neuroimage.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton MC. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 56.Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2011. 10.1145/1961189.1961199.
- 57.Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol Assess. 2013;25:631–42. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 59.Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2020:560623. https://doi.org/https://pubmed.ncbi.nlm.nih.gov/32424236/ [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 60.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bangma DF, Fuermaier ABM, Tucha L, Tucha O, Koerts J. The effects of normal aging on multiple aspects of financial decision-making. PLoS ONE. 2017;12:e0182620. doi: 10.1371/journal.pone.0182620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohr PNC, Li S-C, Heekeren HR. Neuroeconomics and aging: neuromodulation of economic decision making in old age. Neurosci Biobehav Rev. 2010;34:678–88. doi: 10.1016/j.neubiorev.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Samanez-Larkin GR, Knutson B. Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci. 2015;16:278–89. doi: 10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han SD, Boyle PA, Yu L, Fleischman DA, Arfanakis K, Leurgans S, et al. Financial literacy is associated with medial brain region functional connectivity in old age. Arch Gerontol Geriatr. 2014;59:429–38. doi: 10.1016/j.archger.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Ma N, Liu Y, He X-S, Sun D-L, Fu X-M, et al. Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci. 2013;33:4886–95. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai H, Chen J, Liu S, Zhu J, Yu Y. Brain functional connectome-based prediction of individual decision impulsivity. Cortex. 2020;125:288–98. doi: 10.1016/j.cortex.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry. 2019;19:247. doi: 10.1186/s12888-019-2222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheehy-Skeffington J. The effects of low socioeconomic status on decision-making processes. Curr Opin Psychol. 2020;33:183–8. doi: 10.1016/j.copsyc.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 69.Chan MY, Na J, Agres PF, Savalia NK, Park DC, Wig GS. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc Natl Acad Sci. 2018;115:E5144–53. doi: 10.1073/pnas.1714021115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burzynska AZ, Ganster DC, Fanning J, Salerno EA, Gothe NP, Voss MW, et al. Occupational physical stress is negatively associated with hippocampal volume and memory in older adults. Front Hum Neurosci. 2020;14:266. doi: 10.3389/fnhum.2020.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer KE, Riddle NC. Sex differences in aging: genomic instability. J Gerontol A Biol Sci Med Sci. 2018;73:166–74. doi: 10.1093/gerona/glx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, et al. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015;36:1524–35. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013;6:108–17. doi: 10.1016/j.brs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J. Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology. 2016;41:1395–403. doi: 10.1038/npp.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunlop K, Woodside B, Lam E, Olmsted M, Colton P, Giacobbe P, et al. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. NeuroImage Clin. 2015;8:611–8. doi: 10.1016/j.nicl.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–98. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuro-Psychopharmacology. Biol Psychiatry. 2018;81:105–13. doi: 10.1016/j.pnpbp.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Brown V, Peciña M. Neuroimaging studies of antidepressant placebo effects: challenges and opportunities. Front Psychiatry. 2019;10:669. doi: 10.3389/fpsyt.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J, Li W, Miao W, Dai D, Hua J, He H. Age estimation using cortical surface pattern combining thickness with curvatures. Med Biol Eng Comput. 2014;52:331–41. doi: 10.1007/s11517-013-1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kondo C, Ito K, Wu K, Sato K, Taki Y, Fukuda H, et al. An age estimation method using brain local features for T1-weighted images. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:666–9. doi: 10.1109/EMBC.2015.7318450. [DOI] [PubMed] [Google Scholar]
- 81.Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115–24. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 82.Ball G, Adamson C, Beare R, Seal ML. Modelling neuroanatomical variation during childhood and adolescence with neighbourhood-preserving embedding. Sci Rep. 2017;7:17796. doi: 10.1038/s41598-017-18253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aycheh HM, Seong J-K, Shin J-H, Na DL, Kang B, Seo SW, et al. Biological brain age prediction using cortical thickness data: a large scale cohort study. Front Aging Neurosci. 2018;10:252. doi: 10.3389/fnagi.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.