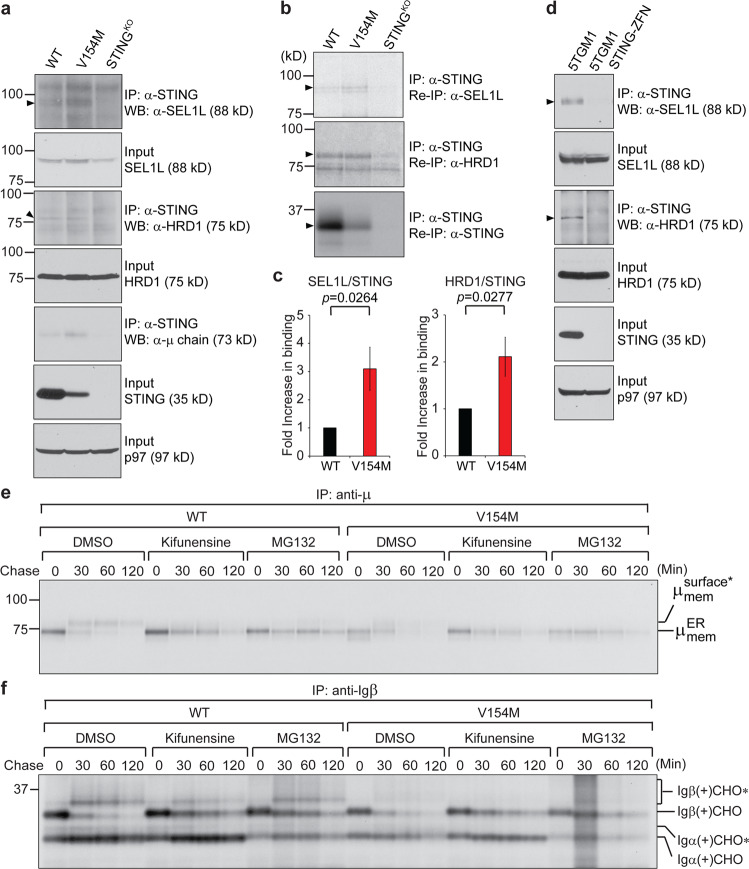
Fig. 4.
The STING V154M mutant protein increased its binding to SEL1L and HRD1 to mediate rapid degradation of the BCR. a B cells purified from WT, V154M and STING-deficient mice were stimulated with LPS for 3 days, lysed in 1.25% digitonin lysis buffer, and analyzed by immunoblotting for SEL1L, HRD1, STING, and p97. An anti-STING antibody was used to immunoprecipitate STING from the same lysates (equal protein amounts), and the immunoprecipitates were analyzed for the presence of SEL1L, HRD1, and Ig μ heavy chains by immunoblotting. b WT, V154M, and STING-deficient B cells were stimulated with LPS for 3 days, starved in cysteine- and methionine-free medium for 1 h, radiolabeled for 4 h, and lysed in 1.25% digitonin lysis buffer. The lysates were immunoprecipitated for STING, and the immunoprecipitates were subsequently boiled in 1% SDS containing 5 mM DTT and diluted with buffer containing NP-40 so that the SDS concentration was 0.05% and the NP-40 concentration was 0.5%. The diluted immunoprecipitates were divided equally into three parts, reimmunoprecipitated with an anti-STING, anti-SEL1L, or anti-HRD1 antibody, and analyzed by SDS-PAGE and autoradiography. c Densitometric quantitation of each radiolabeled protein band in (b) was achieved using phosphorimaging. The data are shown as the ratios of SEL1L/STING and HRD1/STING (means ± SEM). d 5TGM1 and 5TGM1 STING-ZFN (STING-deficient) cells were lysed in 1.25% digitonin lysis buffer and analyzed by immunoblotting for SEL1L, HRD1, STING and p97. An anti-STING antibody was used to immunoprecipitate STING from the same lysates (equal protein amounts), and the immunoprecipitates were analyzed for the presence of SEL1L and HRD1. e, f B cells from WT and V154M mice were stimulated with LPS for 3 days, starved in cysteine- and methionine-free medium for 1 h in the presence of kifunensine (50 µM) or MG132 (50 µM), radiolabeled for 15 min, and chased for 2 h in the presence of kifunensine or MG132. The cells were lysed in Triton X‐114, and the lysates were subjected to phase separation. Membrane‐bound IgM was immunoprecipitated from Triton X‐114‐associated protein fractions using an anti‐μ antibody (e). Igβ was directly immunoprecipitated from Triton X-114 lysates (f). The asterisk denotes endo‐H‐resistant complex glycans. CHO and CHO* indicate high mannose-type glycans and complex-type glycans, respectively