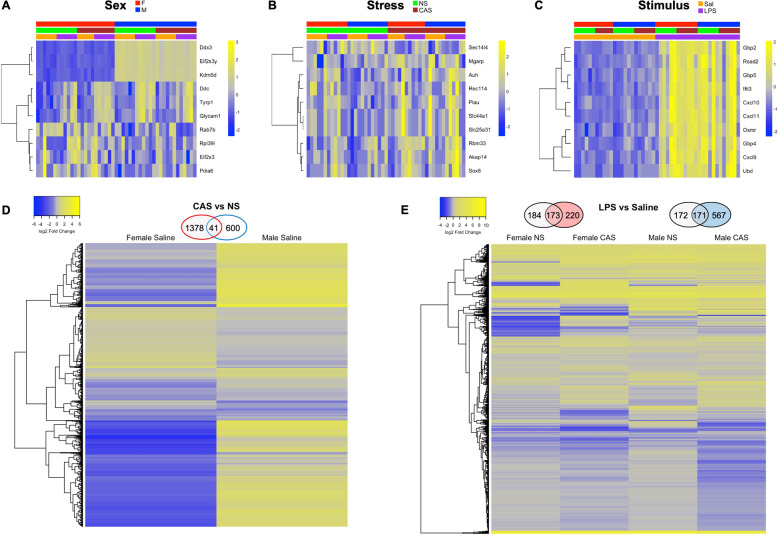
Fig. 2. Chronic adolescent stress (CAS)-associated genes were largely distinct between females and males at baseline.
Female and male rats of NS or CAS background received a systemic injection of either saline or LPS in adulthood. Four hours later, hippocampal tissue was collected for bulk RNA-Seq. Differential expression analysis on the hippocampal transcripts was performed using the package edgeR in Bioconductor. A–C Expression of top ten DEGs are shown for the main factors of sex, stress, and LPS. D The impact of CAS on baseline (unstimulated) gene expression was suassessed within each sex using the contrasts “F.CAS.Sal versus F.NS.Sal” and “M.CAS.Sal versus M.NS.Sal.” The number of differentially expressed genes (DEG) from each paired contrast is displayed in Venn diagrams above the heatmaps. CAS-associated genes were largely distinct between males and females with a greater number of upregulated genes in female rats and similar numbers of downregulated genes across males and females. Subsequent pathway analyses showed that genes differentially regulated by CAS in female rats enriched pathways related to signal transduction, histone deacetylases, and cytoskeleton remodeling. Genes differentially regulated by CAS in male rats were related to G protein-coupled receptor signaling, Notch signaling, and NMDA receptor trafficking (Tables S5–6). E LPS-induced changes in gene expression were assessed within each of the four groups (Female-NS, Female-CAS, Male-NS, Male-CAS) using the contrast “LPS versus Sal.” Similar clusters of genes were differentially regulated by LPS across the four groups, with substantial DEG overlap between NS and CAS conditions within each sex. See GSEA pathway results in detail in Table 1, S7–10 and Fig S1. CAS Chronic Adolescent Stress, NS Non-stressed, Sal Saline.