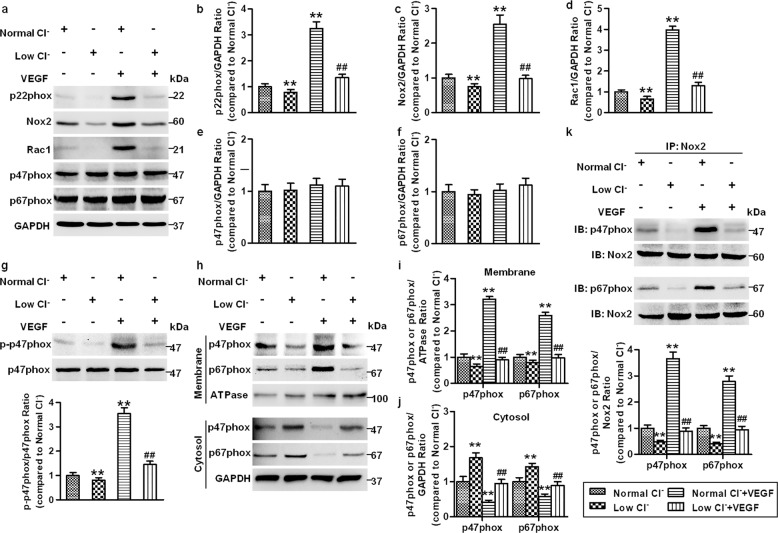
Fig. 5. Reduced [Cl−]i decreases the formation of NADPH oxidase complex.
a HUVECs were cultured in normal Cl− and low Cl− medium containing VEGF (50 ng/mL) for 48 h. The expression of the NADPH oxidase subunits Nox2, p22phox, p47phox, p67phox, and Rac1 was measured in HUVECs. GAPDH was used as a loading control. b–f Quantification of the above protein abundance. **P < 0.01 vs. normal Cl−, ##P < 0.01 vs. normal Cl− + VEGF, n = 5. g Cells in normal Cl− and low Cl− medium were treated with or without VEGF (50 ng/mL) for 30 min. P47phox phosphorylation and total protein expression were examined. **P < 0.01 vs. normal Cl−; ##P < 0.01 vs. normal Cl− + VEGF, n = 6. h After the treatment described in (a), the contents of p47phox and p67phox in the membrane and cytoplasm were detected. Na+/K+ ATPase was used as an internal control of the membrane. Quantification of the abundance of p47phox and p67phox in the membrane (i) and cytoplasm (j). **P < 0.01 vs. normal Cl−; ##P < 0.01 vs. normal Cl− + VEGF, n = 5. k Cell lysates were immunoprecipitated with anti-Nox2 and immunoblotted with anti-p47phox or anti-p67phox. **P < 0.01 vs. normal Cl−; ##P < 0.01 vs. normal Cl− + VEGF, n = 5.