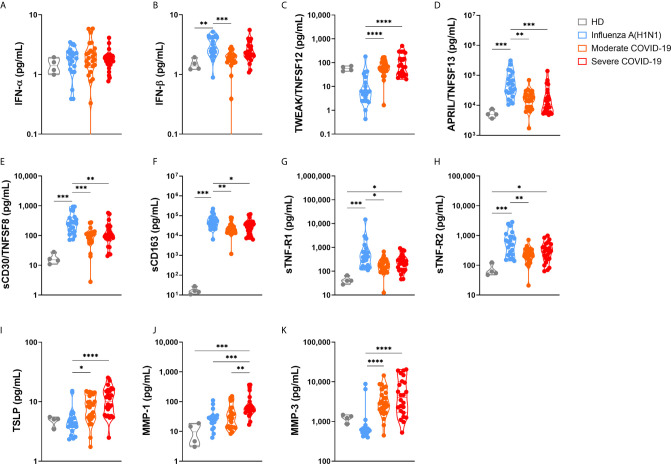
Figure 8.
Immune mediators in the plasma of patients with pandemic influenza A(H1N1) and COVID-19. Levels of different soluble immune mediators in plasma samples from patients with COVID-19 (n=25 moderate, 24 severe) and pandemic influenza A(H1N1) (n=23), as well as in samples from healthy volunteer donors (HD, n=4) were assessed by Luminex assay. Violin plots display medians and interquartile ranges (IQR). Differences between groups we estimated using the Kruskal-Wallis test with post hoc Dunn´s test. Significant differences are denoted by bars and asterisks: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. (A) IFN-α, interferon-alpha; (B) IFN-β, interferon-beta; (C) TWEAK/TNFSF12, tumor necrosis factor-like weak inducer of apoptosis/tumor necrosis factor ligand superfamily member 12; (D) APRIL/TNFSF13, A proliferation-inducing ligand/tumor necrosis factor ligand superfamily member 13; (E) sCD30/TNFRSF8, soluble CD30/tumor necrosis factor ligand superfamily member 8; (F) sCD163, soluble CD163; (G) sTNF-R1, soluble tumor necrosis factor receptor 1; (H) sTNF-R2, soluble tumor necrosis factor receptor 2; (I) TSLP, thymic stromal lymphopoietin; (J) MMP-1, metalloprotease 1; (K) MMP-3, metalloprotease 3.