Brolucizumab (Beovu, Novartis, Basel, Switzerland) is the newest anti-vascular endothelial growth factor (anti-VEGF) drug. lt was approved for the treatment of neovascular age-related macular degeneration (nAMD) by the US-FDA on October 7, 2019, followed by the European Commission’s approval for use in the European Union on February 13, 2020. Brolucizumab received marketing approval based on the two pivotal phase 3 clinical trials—HAWK and HARRIER, with a q8/q12 week dosing regimen [1]. To the best of our knowledge, real-world clinical data of consecutive patients who have undergone this therapy has not yet been reported. Here, we report the early clinical outcomes regarding safety and efficacy after brolucizumab administration.
A retrospective, consecutive, interventional, uncontrolled, multicenter study was conducted incorporating data from four centers in the United States of America (USA). Institutional Review Board approval was obtained at each participating center and the investigators adhered to the tenets of the Declaration of Helsinki. All patients were treated with at least one intravitreal injection of brolucizumab 6 mg between December 2019 and February 2020. A minimum of 4-weeks follow-up was required to be included in the study. Eyes with structural changes other than nAMD and patients with vitreoretinal interface diseases were excluded. Each patient underwent best-corrected visual acuity (BCVA) measurement with a Snellen chart (converted to LogMAR for analysis), central subfield thickness (CST) with spectral-domain optical coherence tomography and intraocular pressure measurement along with complete ophthalmic examination at baseline and the last follow-up after brolucizumab injection. Descriptive statistics including mean, standard deviation, median, and range were calculated for continuous variables. A paired sample t-test was used to measure the mean differences between preinjection and postinjection values.
Forty-two eyes of 42 patients were included in this study. The patients received a total of 60 injections. The mean age was 79.2 ± 7.0 years and 57.2% were females. The mean follow-up period was 7.2 ± 3.6 weeks after the first injection of brolucizumab. Twenty-nine eyes received a single injection of brolucizumab, nine eyes received two injections, three eyes had three injections, and one eye had four injections. All the eyes were previously treated with single or a combination of other intravitreal anti-VEGFs (bevacizumab, ranibizumab, and aflibercept). The median number of previous anti-VEGF injections was 19 (range 3–107). Fifteen eyes received aflibercept and one eye received ranibizumab during the follow-up after brolucizumab injection. Immediate data prior to the first brolucizumab injection was considered as the baseline, and the subsequent data after brolucizumab injection were included in the analysis.
Key outcomes
Visual acuity
Mean BCVA at baseline was 0.42 ± 0.28 LogMAR (20/50) and was 0.36 ± 0.29 (20/50) at the last follow-up p = 0.33 (95% CI = −0.0637 to 0.1837).
Disease activity
Mean CST at baseline was 314 ± 94 microns which improved significantly to 263 ± 51 microns (p = 0.0027; 95% CI = 18.17–83.83). At baseline, sub-retinal fluid (SRF) was present in 38 eyes (90.4%). SRF completely resolved in 15 eyes (39.4%), was reduced in 17 eyes (44.7%), and persisted without any change in 6 eyes (15.7%). Intra-retinal fluid (IRF) was present in 19 (45.2%) eyes at baseline and completely resolved in 7 eyes (36.8%), decreased in 8 eyes (42.1%), and persisted without any change in 4 eyes (21.0%). Pigment epithelial detachment (PED) was present in 31 eyes (73.8%) at baseline and resolved in 2 eyes (6.4%), was reduced in 13 eyes (41.9%) and did not show any change in 16 eyes (51.6%) (Fig. 1). To avoid the probable error due to pooled analysis of all the patients, we performed a sub-analysis of patients who underwent a single injection of brolucizumab (29). There was no statistically significant difference in any of the parameters. This could be due to a limited number of patients with multiple injections. Patients who underwent more than one injection of brolucizumab received it at an interval of 4–6 weeks and the mean number of injections was 1.4 ± 0.73.
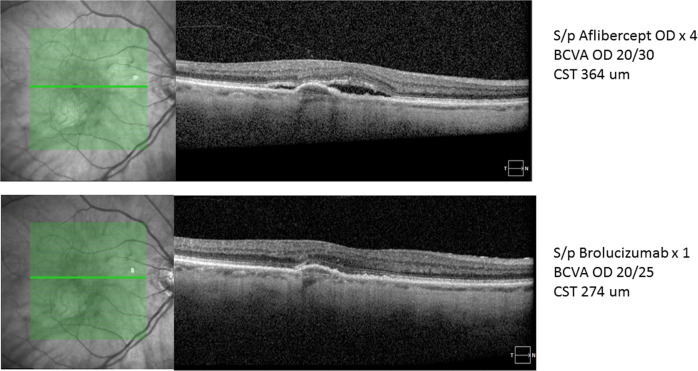
Fig. 1. Representative case of nAMD showing reduction of central subfield thickness (CFT) from 364 to 274 um along with the complete resolution of sub-retinal fluid (SRF) and reduction in pigment epithelial detachment (PED) height after 6 weeks of brolucizumab first injection.
Furthermore, BCVA improved from 20/30 to 20/25. Patient was previously treated with four injections of aflibercept. (Courtesy: SRS).
Safety
The occurrence of ocular or systemic adverse events was noted at each follow-up visit after brolucizumab treatment. In particular, the presence of anterior and posterior segment inflammation was recorded with a special emphasis on the development of retinal occlusive vasculitis at any point of time after brolucizumab injection. In this study, none of the sites reported any signs of inflammation, vasculitis, or any other ocular or systemic adverse effects in any of their cases.
To summarize, early real-world data in this limited series demonstrated that brolucizumab was safe and effective in stabilizing BCVA in patients who have undergone previous treatment with other anti-VEGFs agents. Tachyphylaxis is a phenomenon wherein repeat administration of a drug is associated with a decreased therapeutic response. However, it could not be determined in this study due to the history of multiple drug usage and wide range (3–107) of injections. Brolucizumab also appears to be effective in further reducing the fluid in different compartments (SRF/IRF). Also, a reduction in PED was noted in approximately half of the cases. This study was not intended to determine the effect of switching to brolucizumab. The American Society of Retina Specialists (ASRS) released an alert on February 23 and March 30, 2020, reporting 11 and 21 cases of occlusive vasculitis, respectively [2]. In our study, we did not find any case of anterior or posterior segment inflammation including occlusive retinal vasculitis. However, given the reported rates, our series is too small to make any definitive statements regarding safety. Intraocular inflammation has been reported with other anti-VEGFs in the past, most notably with aflibercept [3]. Postinjection retinal vasculitis is a newer phenomenon for anti-VEGF agents used for intravitreal injections. Few authors have published cases with occlusive vasculitis after brolucizumab in the recent past [4–6]. There are few hypotheses proposed to explain this immunogenic phenomenon, but it is not clear at this point due to the paucity of cases with detailed analysis [6, 7].
There are limitations to the study due to its small sample size, absence of a control group, and short follow-up. Furthermore, all the eyes were previously treated with a significant number of other anti-VEGF injections. Hence, these results cannot be directly extrapolated to the treatment naive eyes. Studies with large sample size and long-term follow-up with a comparison arm will be important to better understand the anatomic efficacy and durability benefits of brolucizumab and the risks of occlusive vasculitis which will be critical to define the optimal use of brolucizumab in the real world.
Compliance with ethical standards
Conflict of interest
AS: CONSULTANT: for Novartis, Allergan, Bayer and Intas; SRS reports and Consultant: Amgen, Allergan, Novartis, Regeneron, Roche/Genentech, 4DMT. Merck, Optos, Centervue, Heidelberg; Research Instruments: Topcon, Nidek, Carl Zeiss Meditec, Optos, Centervue, Heidelberg. JH’s disclosures are as follows: Consulting: 4DMT, Adverum, Aerie, Aerpio, Aldeyra, Allegro, Alzheon, Annexon, Apellis, Asclepix, Beaver-Visitec, Galimedix, Genentech, Gyroscope, iRenix, jCyte, Kala, Kanghong, NGM, Notal Vision, Novartis, Ocugenix, Oculis, Ocunexus, Ocular Therapeutix, Palatin, Pfizer, Regeneron, Regenxbio, Santen, Scifluor, Shire, Stealth, Tyrogenex, Voyant; Research: Aerie, Aerpio, Apellis, Genentech, Graybug, Gyroscope, Hemera, Janssen R&D, KalVista, Kanghong, Novartis, Ophthotech, Optovue, Regeneron, Regenxbio, Stealth; Equity: Adverum, Aldeyra, Allegro, Aviceda, Digital Surgery Systems, jCyte, Ocular Therapeutix; DB reports and Consultant: Genentech, Allergan, Roche, Regeneron, Bayer, Novartis. PK reports and Consultant: Allergan, Bayer, Novartis, Kanghong, Kodiak, RegenexBio, Regeneron. The other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/15/2020
A Correction to this paper has been published: 10.1038/s41433-020-01351-7
References
- 1.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Retina Specialists Beovu update for ASRS members. https://www.asrs.org/clinical/clinical-updates/1968/Beovu-Update. 2020.
- 3.Souied E, Dugel P, Ferreira A, et al. Severe ocular inflammation following ranibizumab or aflibercept injections for age-related macular degeneration: a retrospective claims database analysis. Ophthalmic Epidemiol. 2016;23:71–9. doi: 10.3109/09286586.2015.1090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haug SJ, Hien DL, Uludag G, Ngoc TTT, Lajevardi S, Halim MS, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi: 10.1016/j.ajoc.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain A, Chea S, Matsumiya W, Halim MS, Yaşar Ç, Kuang G, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18:100687. doi: 10.1016/j.ajoc.2020.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John VJ, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020. S0161–6420;30371–7. 10.1016/j.ophtha.2020.04.017. [Online ahead of print]. [DOI] [PubMed]
- 7.Sharma A, Kumar N, Parachuri N, Sharma R, Bandello F, Kuppermann BD, et al. Brolucizumab and immunogenicity. Eye. 2020. http://www.nature.com/articles/s41433-020-0853-9 [DOI] [PMC free article] [PubMed]