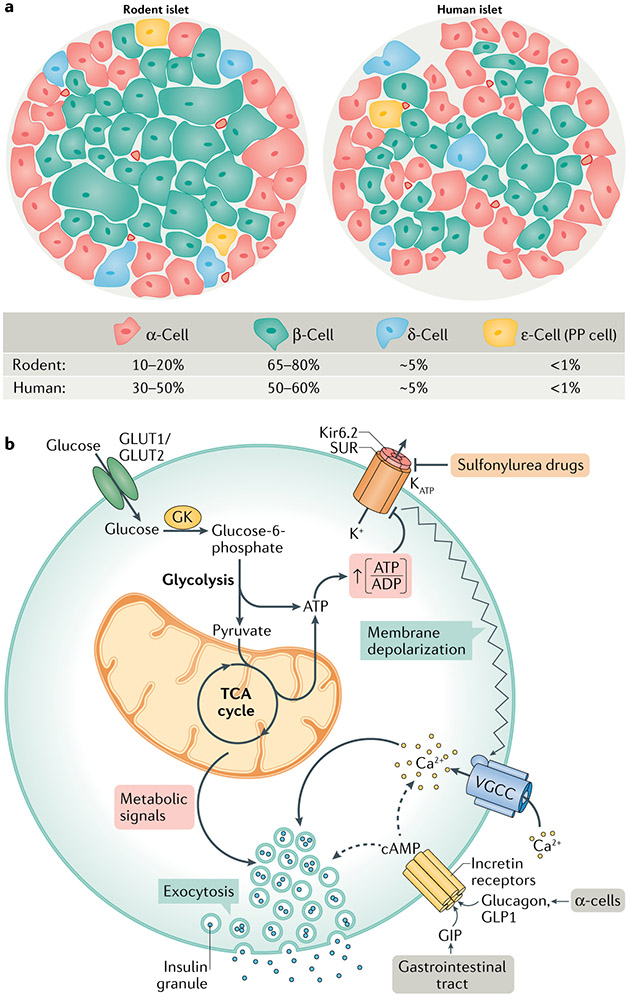
Fig. 1 ∣. Islet cell architecture and fundamental signalling pathways of GSIS.
a ∣ Comparison of the cellular composition of rodent and human islets. Rodent islets are made up of 10–20% of α-cells, found predominantly on the outer mantle, and 65–80% of β-cells comprising their inner core. δ-cells, γ-cells (also known as or pancreatic polypeptide (PP) cells) and ε-cells are found scattered throughout the islet. Human islets contain a higher percentage of α-cells, which are found throughout the islet, and a slightly lower percentage of β-cells than rodents. b ∣ Glucose-stimulated insulin secretion (GSIS) is mediated by a triggering pathway (solid arrows) and amplification pathways (dashed arrows). Glucose uptake into β-cells occurs via GLUT1 (human) or GLUT2 (rodent) transporters; glucose uptake is not rate limiting for GSIS, but rather the rate of β-cell glucose metabolism is controlled by glucokinase (GK), which determines the entry of glucose into the glycolytic pathway, followed by its oxidation via the tricarboxylic acid (TCA) cycle and subsequent generation of ATP. Glucose metabolism initiates the triggering pathway of GSIS via an elevation in the ATP to ADP ratio, resulting in the closure of KATP channels, membrane depolarization and subsequent opening of voltage-gated calcium channels (VGCC). The resulting increase in intracellular calcium drives the triggering phase of insulin granule exocytosis. The importance of GK and the KATP channel for regulating first-phase insulin secretion is clearly illustrated by the functional impact of human genetic mutations in these proteins. Human GK mutations that lower its Km result in a form of persistent hyperinsulinism and hypoglycaemia caused by a lower glucose threshold for the activation of β-cell glucose metabolism and insulin secretion152,153. Other GK mutations cause partial or complete loss of enzyme activity, resulting in insulin insufficiency and in a form of maturity onset diabetes of the young (MODY2)152,153. Islet cell KATP channels are composed of an octamer of four potassium channel subunits (Kir6.2) and four sulfonylurea receptor 1 (SUR1) regulatory subunits, which bind ATP to cause channel closure, plasma membrane depolarization and subsequent activation of insulin secretion. KATP channel closure can also be induced by sulfonylurea drugs, which are used as anti-diabetic medications. Mutations that force the chronic closure of the channel result in hypoglycaemia due to insulin hypersecretion154. Conversely, gain-of-function mutations that prevent closure of the channel by ATP cause syndromes of insulin insufficiency and neonatal diabetes mellitus155,156. The amplification pathways are dependent upon membrane depolarization initiated by the triggering pathway. Multiple metabolic signalling mechanisms contribute to the amplification of insulin secretion, as detailed in subsequent figures. cAMP, cyclic AMP; GIP, gastric inhibitory polypeptide; GLP1, glucagon-like peptide 1.