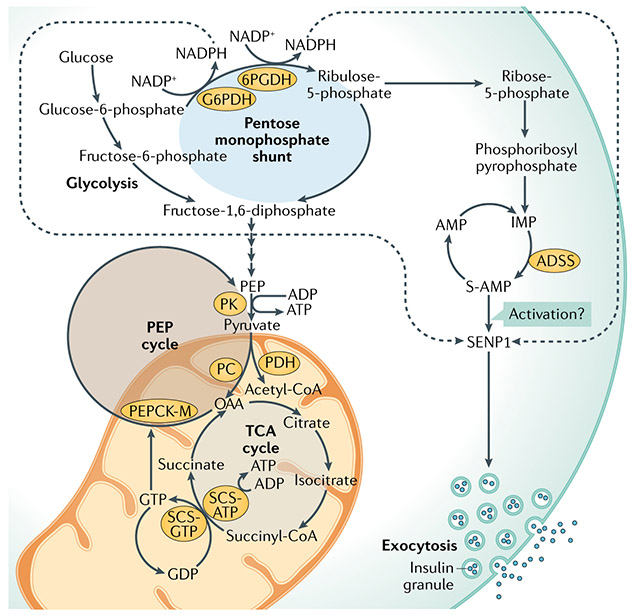
Fig. 3 ∣. Pentose monophosphate shunt, phosphoenolpyruvate cycle and their nucleotide metabolites in GSIS.
As highlighted in the article, the regulation of glucose-stimulated insulin secretion (GSIS) may involve the synthesis of nucleotides other than ATP, specifically adenylosuccinate (S-AMP) generated by the pentose monophosphate shunt or GTP produced in the tricarboxylic acid (TCA) cycle by the GTP-forming isoform of the TCA cycle enzyme succinyl-CoA synthetase (SCS-GTP). Glucose metabolism via the pentose monophosphate shunt generates ribose-5-phosphate and inosine monophosphate (IMP), which is converted to S-AMP via adenylosuccinate synthetase (ADSS). The inhibition of ADSS to block S-AMP formation inhibits GSIS, while infusion of S-AMP into patch-clamped human β-cells amplifies insulin granule exocytosis. NADPH generated in the first two reactions of the pentose monophosphate shunt catalysed by glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) may synergize with NADPH generated by IDH1 to enhance insulin granule exocytosis via the activation of sentrin/SUMO-specific protease 1 (SENP1) (FIG. 2). The effects of S-AMP on granule exocytosis are attenuated in β-cells with the suppression of SENP1 but the mechanisms by which S-AMP may interact with SENP1 remain unknown (question mark). Alternatively, succinyl-CoA can be converted to succinate in the TCA cycle by SCS-GTP. The manipulation of SCS-GTP expression affects GSIS via the modulation of GTP levels, required for activity of the mitochondrial isoform of phosphoenolpyruvate carboxykinase (PEPCK-M). The synthesis of phosphoenolpyruvate (PEP) by this PEPCK-catalysed PEP cycle produces ATP for the regulation of the KATP channel. OAA, oxaloacetate; PK, pyruvate kinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.