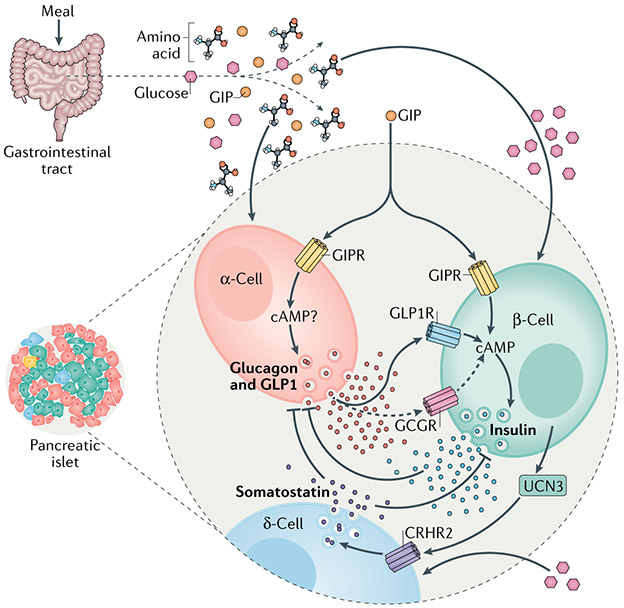
Fig. 5 ∣. Other hormones in control of insulin secretion.
Nutrient ingestion increases the circulating concentrations of glucose, amino acids and the incretin hormones produced by the gastrointestinal tract: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP1). Agonism of either the GIP receptor (GIPR) or GLP1 receptor (GLP1R) potentiates glucose-stimulated insulin secretion (GSIS) through a cyclic AMP (cAMP)-dependent mechanism. GIP also potentiates amino acid-stimulated glucagon secretion in α-cells, which occurs through as-of-yet undefined mechanisms. The secretion of glucagon generally opposes the action of insulin and insulin negatively regulates glucagon secretion. However, there is now evidence that endocrine activity of α-cells is important for regulating glucose-stimulated insulin secretion in β-cells. Together with glucagon, α-cells also produce GLP1 through alternative processesing of the proglucagon peptide precursor, and both hormones increase the level of cAMP in β-cells to regulate insulin secretion in response to a meal. This is mostly mediated by binding of these hormones to GLP1R and to a lesser extent (dashed arrows) to the glucagon receptor (GCGR). Thus, the incretin action of GIP to stimulate insulin secretion includes both direct effects on β-cells and indirect actions on α-cells, mediated by the paracrine stimulatory effects of proglucagon-derived peptides. Urocortin 3 (UCN3) produced by β-cells initiates a separate mechanism of paracrine interaction within the islet. UCN3 enhances δ-cell activity to increase somatostatin secretion through the corticotropin-releasing hormone receptor(CRHR2). The elevation in somatostatin secretion inhibits the secretory activity of β-cells, completing a negative feedback loop.