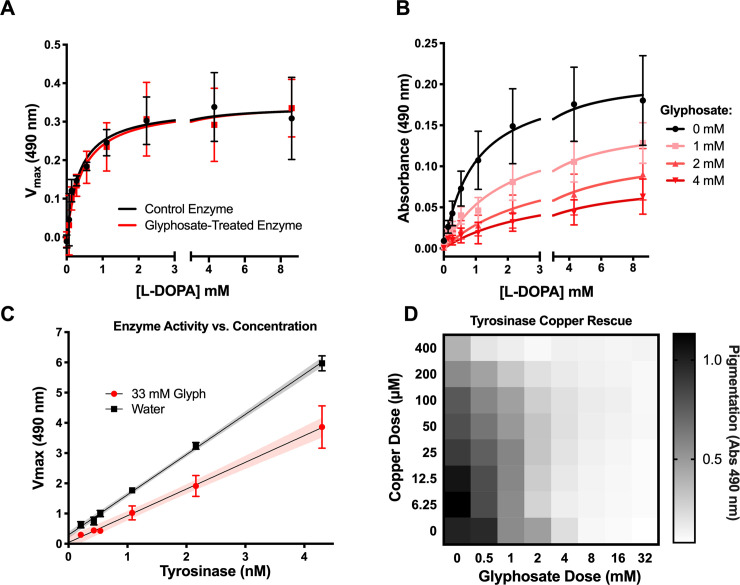
Fig 6. Glyphosate does not directly inhibit tyrosinase activity.
(A). Tyrosinase activity is not irreversibly inhibited, and glyphosate-treated enzyme has normal activity when glyphosate is dialyzed out of solution. (B) Glyphosate appears as a noncompetitive inhibitor of tyrosinase in Michaelis–Menten kinetics assays measuring the change in absorbance at 490 nm over 24 hours compared to the no tyrosinase background. (C) The rate of dopachrome formation with glyphosate treatment is smaller than the slope of the control treatment across all concentrations of tyrosinase. This reduced slope indicates reversible inhibition. The assay is performed under constant L-DOPA and glyphosate concentrations. Shaded areas represent the 95% CI of the linear regression. (D) Adding Cu+2 to L-DOPA-tyrosinase reactions with glyphosate does not rescue melanin inhibition compared to the glyphosate-free control. (See also S5 Fig) Grayscale bars represent mean absorbance at 490 nm relative to no glyphosate and no copper control. The darker colors correspond to increased pigment formation. Error bars in (A–C) represent ±SD. Each experiment represents at least 3 independent replicates. For underlying data, please see Data Availability section and/or S1 Table. L-DOPA, 3,4-dihydroxyphenylalanine.