Figure 3.
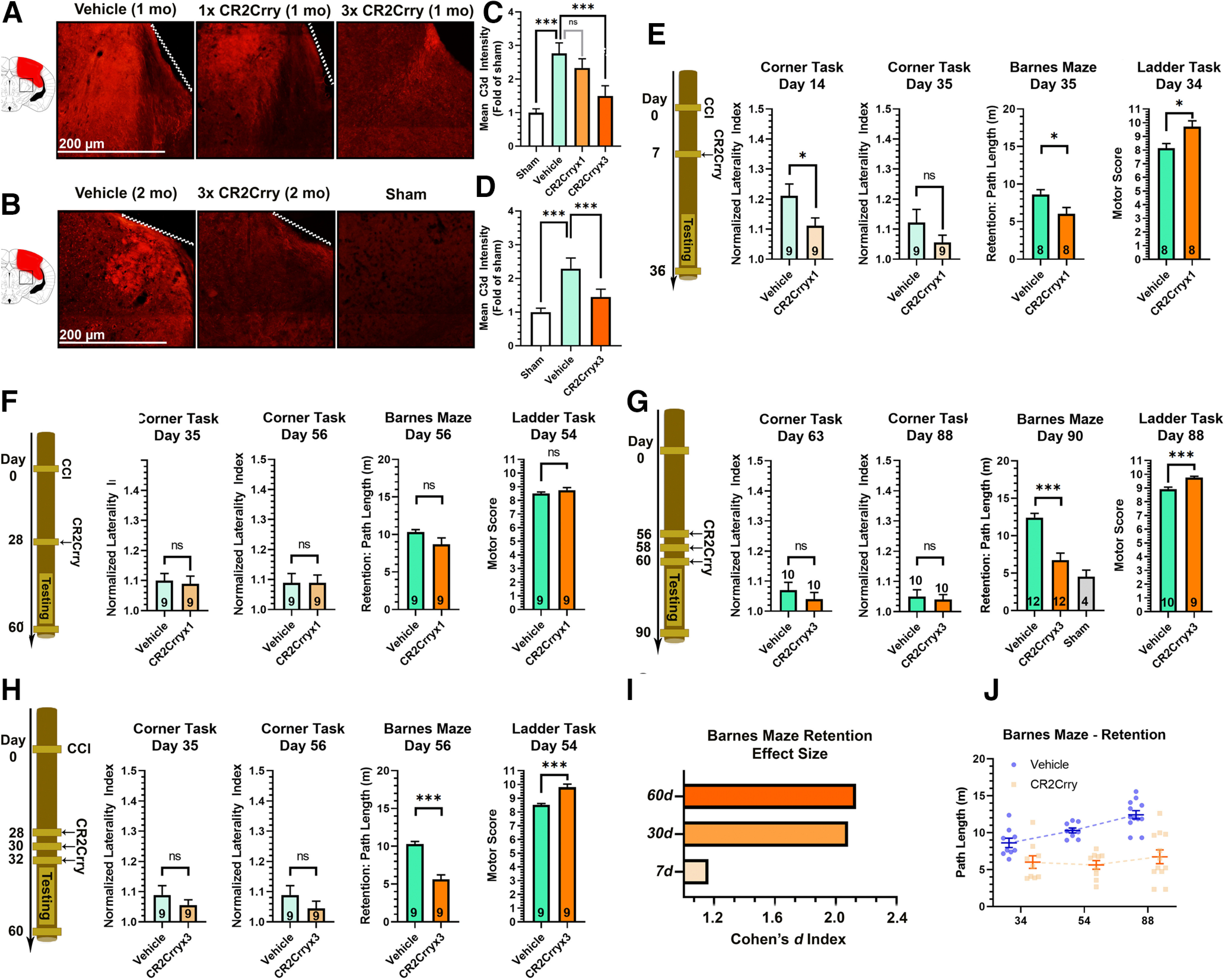
Chronically administered CR2Crry suppresses C3d deposition in the brain after TBI. A, B, IF staining for C3d deposition in the perilesional brain following TBI and administration of CR2Crry or vehicle. A, Mice were treated at 30 d after TBI with a single dose of PBS vehicle of CR2Crry, or with 3 doses of CR2Crry every 48 h. C3d deposition was measured 1 week after initiation of treatment. B, Mice were treated at 2 months after TBI with 3 doses of PBS or CR2Crry every 48 h. C3d deposition was measured 1 week after initiation of treatment. Fields are 250 µm × 250 µm, representative images. C, D, Quantification of C3d deposition in A, B, using mean IF intensity compared with sham using unbiased stereology. N = 5 animals/group (2 or 3 slices per mouse). ***p < 0.001 (ANOVA with Bonferroni). E–H, Motor and cognitive outcomes measures, as indicated, after vehicle or CR2Crry treatment. E, Single-dose CR2Crry treatment at 7 d after TBI. F, Single-dose CR2Crry treatment at 28 d after TBI. G, Three doses of CR2Crry treatment every 48 h starting 28 d after TBI. H, Three doses of CR2Crry treatment every 48 h starting 56 d after TBI. Student's t test. N values shown on bars. *p < 0.05. ***p < 0.001. I, Effect size of CR2Crry treatment on Barnes maze performance compared with vehicle at different time points of administration measured via the Cohen's d index. J, Change in path length on retention day at different time points after TBI between vehicle and CR2Crry-treated animals. N = 8-12/group. ns = p > 0.05.
