Figure 2.
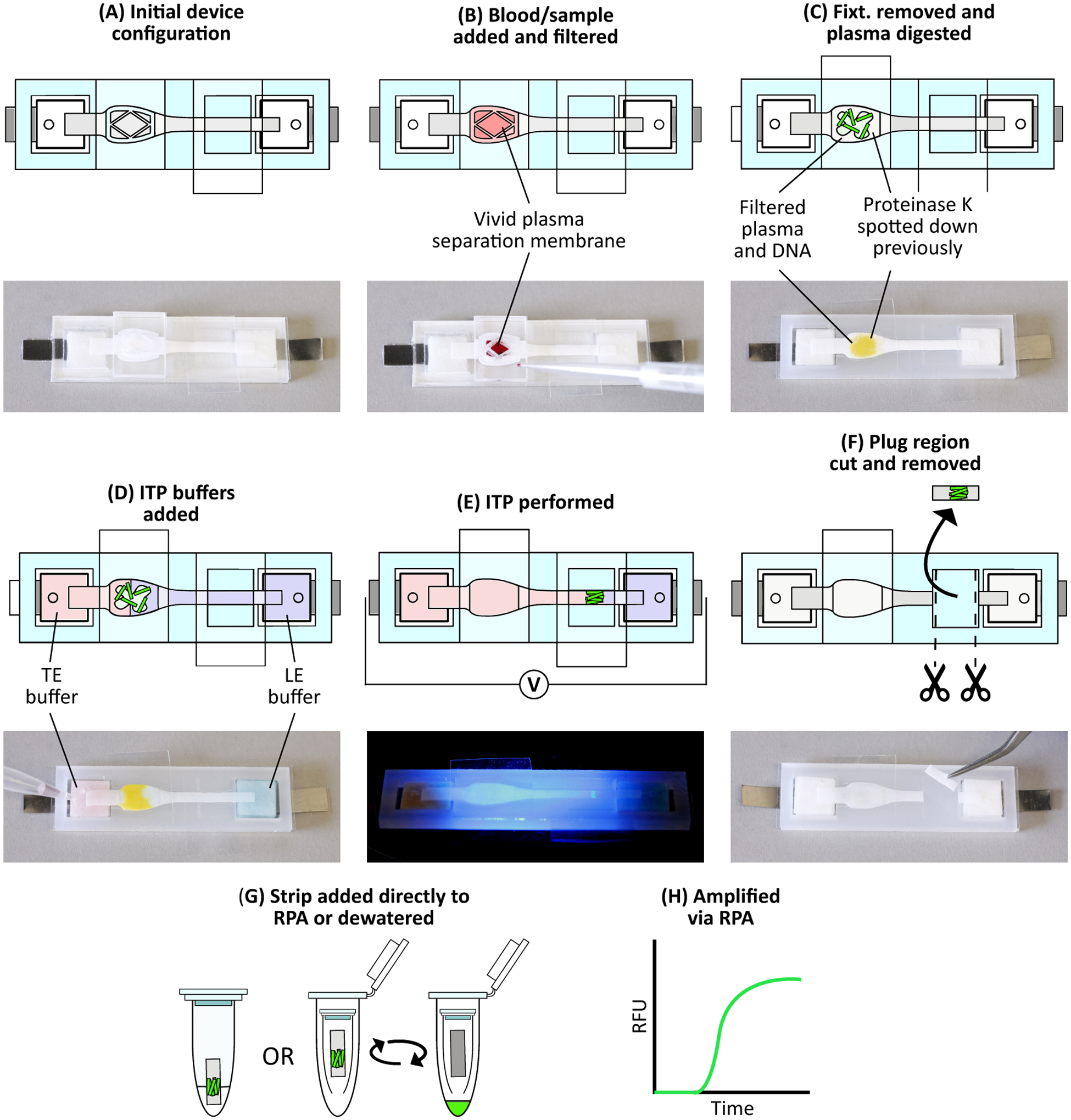
Device protocol to extract DNA from whole blood. (A) Initial device configuration. (B) We add 33 μL whole blood spiked with target DNA and fluorescently labeled tracking DNA onto the Vivid membrane. Blood filtration occurs for 4 minutes, during which plasma and nucleic acids filter through to the Fusion 5 membrane. (C) After filtration, we remove the blood filtration fixturing and Vivid and place a piece of PCR tape over the sample port. Proteolysis then occurs for 15 minutes. (D) We add ITP buffers to their respective reservoirs. Color is added to the image for clarity: red represents trailing electrolyte buffer while blue represents leading electrolyte buffer. (E) Constant current is applied across the device, beginning ITP and focusing the DNA into a narrow plug which then migrates towards the leading electrolyte reservoir. We monitor this process via fluorescent microscopy. (F) Once the ITP plug reaches the eluate port, we stop ITP, remove the eluate port tape, and cut out the exposed portion of Fusion 5 membrane containing the ITP plug. Buffer colors are removed for clarity. (G) We add the strip directly to an RPA reaction, or first dewater the strip using a centrifuge and then add the resulting liquid sample to an RPA reaction. (H) RPA is then performed with the real-time fluorescence recorded.
