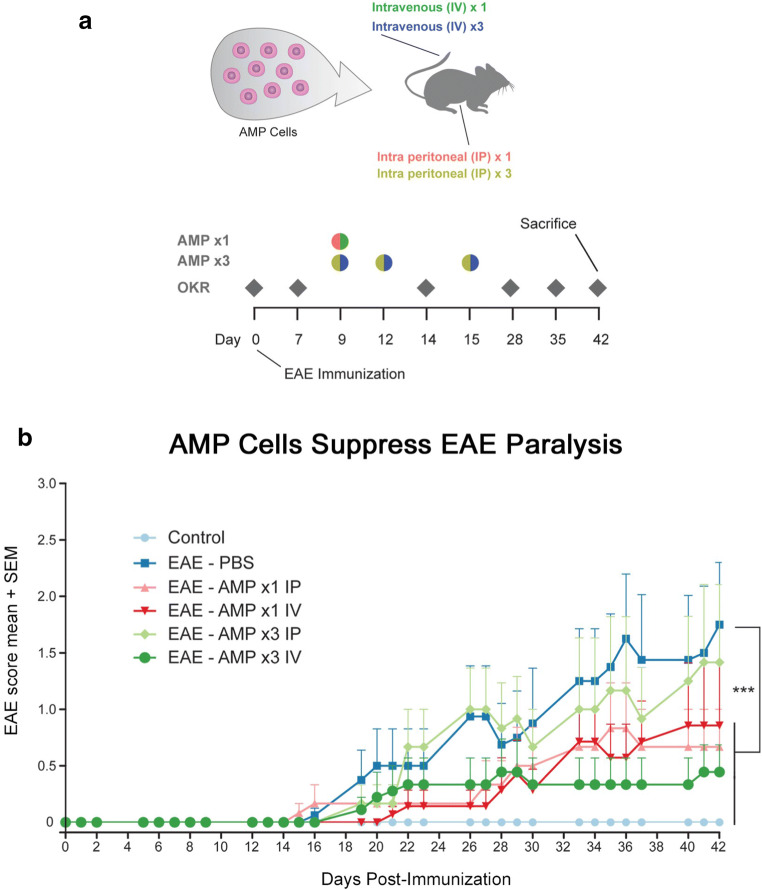
Fig. 1.
AMP cells suppress EAE induced ascending paralysis. a Experimental design is diagrammed: A single dose (1×) of 2 × 106 AMP cells were given by intravenous (IV) or intraperitoneal (IP) injection on day 9 after immunization (p.i.). Additional groups of EAE mice were treated with 3 doses (3×) of 1 × 106 AMP cells given IP or IV on days 9, 12, and 15 p.i. OKR was measured at baseline (day 0) before immunization, and weekly thereafter. EAE mice were scored daily for signs of ascending paralysis, and mice were euthanized on day 42 p.i. b C57BL/6 mice were immunized with MOG on day 0, and daily EAE clinical scoring was done based on a 5 point scale. The clinical symptoms of EAE began by day 14 p.i. and progressed through day 42 in vehicle (PBS)-treated EAE mice (N = 6). 1× AMP cells given by IV administration (N = 7), and 3× AMP cell treatments given by IP (N = 6) or IV (N = 9) injection all showed a significant (***p < 0.001) attenuation of EAE clinical scores as compared with vehicle-treated EAE mice, whereas 1× treatment with AMP cells by IP injection (N = 6) did not significantly reduce EAE scores. Data represent mean ± SEM