Abstract
Aging is a process that can be accompanied by molecular and cellular alterations that compromise cardiac function. Although other metabolic disorders with increased prevalence in aged populations, such as diabetes mellitus, dyslipidemia, and hypertension, are associated with cardiovascular complications; aging-related cardiomyopathy has some unique features. Healthy hearts oxidize fatty acids, glucose, lactate, ketone bodies, and amino acids for producing energy. Under physiological conditions, cardiac mitochondria use fatty acids and carbohydrate mainly to generate ATP, 70% of which is derived from fatty acid oxidation (FAO). However, relative contribution of nutrients in ATP synthesis is altered in the aging heart with glucose oxidation increasing at the expense of FAO. Cardiac aging is also associated with impairment of mitochondrial abundance and function, resulting in accumulation of reactive oxygen species (ROS) and activation of oxidant signaling that eventually leads to further mitochondrial damage and aggravation of cardiac function. This review summarizes the main components of pathophysiology of cardiac aging, which pertain to cardiac metabolism, mitochondrial function, and systemic metabolic changes that affect cardiac function.
Keywords: cardiac aging, fatty acid oxidation, carbohydrate metabolism, ketone bodies, autophagy, mitochondria
Introduction
Cardiac aging is an intrinsic process, accompanied by structural and functional changes of the heart, which increase vulnerability to stress and cardiovascular-related mortality and morbidity. The most common features of cardiac aging include progressive degeneration of myocardium, impaired cardiac conduction system, aortic valvular stenosis and mitral valvular insufficiency, increased wall thickening, and stiffening of the arteries. Aging-related cardiovascular complications also include hypertension, atrial fibrillation, and atherosclerosis.
Aged myocardium presents with left ventricular hypertrophy, increased left ventricular end-diastolic pressure, lower fractional shortening, diastolic dysfunction, fibrosis, cardiomyocyte apoptosis, oxidative stress, inflammation, and lower exercise capacity (Dai et al., 2012). Age-related cardiac complications that lead to heart failure with preserved ejection fraction (HFpEF) have higher prevalence in women than men (Merz and Cheng, 2016). This difference has been attributed to differential dependence of cardiac function on estrogen signaling, which attenuates in post-menopausal women (Mahmoodzadeh et al., 2006).
Many studies have explored the association of cardiac complications with alterations in metabolic pathways (Lopaschuk, 2017). As the world population grows older and cardiovascular complications become more frequent, the involvement of alterations in cardiac metabolism in cardiac aging pathophysiology is warranted. The objective of this article pertains to better understanding of metabolic amendments that occur during cardiac aging including intermediary metabolism and mitochondrial function and may unravel novel factors that account for pathophysiology in aged hearts.
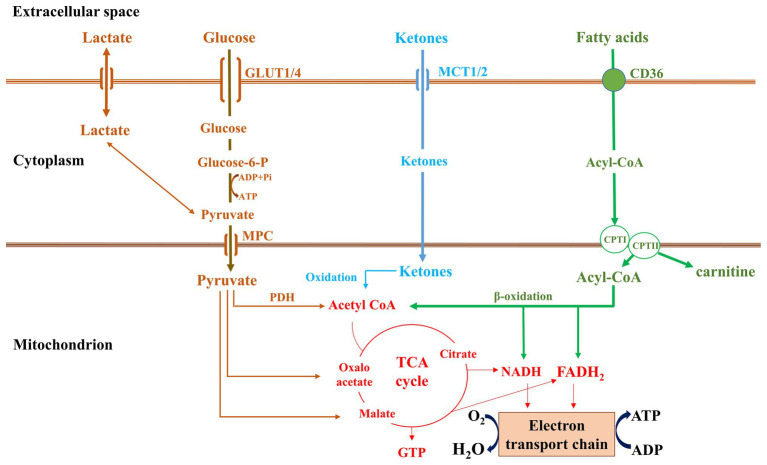
Cardiac Metabolism and Function in the Healthy Heart
A healthy heart pumps about 7,000 litters of blood per day, which requires a significant amount of ATP that is acquired through oxidation of fatty acids, glucose, amino acids, ketone bodies, and lactate. Fatty acid oxidation (FAO) is the primary source for cardiac ATP synthesis (Lopaschuk et al., 2010). Depending on cardiac work load, hormonal status, fasting vs. feeding state and availability of energy substrates, and the ratio of energy supply from different substrates is adjusted accordingly (Ramos-Roman et al., 2012; Viscarra and Ortiz, 2013; Karwi et al., 2018).
Lipoprotein- or adipose tissue-derived free fatty acids are transported into the heart via the transmembrane fatty acid transporter, cluster of differentiation (CD36). For the uptake of lipids from triglyceride-rich lipoprotein, lipoprotein lipase-mediated lipolysis precedes, while whole lipoprotein uptake is also possible (Coburn et al., 2000; Bharadwaj et al., 2010; Son et al., 2018). Fatty acids are then converted to fatty acyl-CoA by acyl-CoA synthase and fatty acylcarnitine by carnitine palmitoyltransferase I (CPT-I)β (Tomec and Hoppel, 1975), which is transported into the mitochondrial matrix, where it is converted back to long-chain acyl-CoA by carnitine palmitoyltransferase II (CPT-II; Hoppel et al., 1998). β-oxidation processes fatty acyl-CoAs to acetyl-CoA yielding NADH and FADH2. Further acetyl-CoA oxidation within the mitochondrial matrix by the tricarboxylic acid cycle (TCA cycle or Krebs cycle) produces three NADHs and one FADH2 and GTP. The NADH and FADH2 are further oxidized by electron transport chain, which leads to ATP generation (Ma and Li, 2015).
Glucose is obtained by cardiomycoytes either from circulation or from hydrolysis of intracellular glycogen stores. Glucose serves as the main cardiomyocyte energy production fuel in the fetal hearts. Glucose uptake by cardiomyocytes occurs through insulin-dependent glucose transporters (GLUT4) or via the insulin-independent GLUT1. On entering the cell, glucose is phosphorylated by hexokinase to glucose-6-phosphate (G-6-P), which is used for glycolysis, glycogen synthesis, the hexosamine biosynthesis pathway, or the pentose phosphate pathway. Pyruvate produced during glycolysis is transferred via mitochondrial pyruvate carrier (MPC) to mitochondrial matrix, where it is converted to acetyl-CoA by pyruvate dehydrogenase (PDH), and enters the TCA cycle (Ma and Li, 2015). PDH complex (PDC) activity is inhibited by PDH kinases (PDKs) that respond to allosteric modifiers derived from glycolysis and FAO. Ketone bodies are produced in the liver during starvation and fasting. There are three types of ketone bodies that the heart uses: acetone, acetoacetate, and β-hydroxybutyrate (βOHB; Cotter et al., 2013). βOHB-dehydrogenase oxidizes βOHB to acetoacetate in mitochondria. Acetoacetate is then converted to acetoacetyl-CoA by the rate limiting enzyme succinyl-CoA:3-oxoacid-CoA-transferase (SCOT). Finally, acetoacetyl-CoA is converted into acetyl-CoA by acetyl-CoA acetyltransferase (Williams et al., 1971) and enters the TCA cycle and electron transport chain for generating ATP (Figure 1).
Figure 1.
Metabolic pathways in normal heart.
Alterations in Lipid Metabolism During Cardiac Aging
Considering the heavy reliance of cardiac ATP synthesis on FAO, it is not surprising that either deprivation or oversupply of fatty acids can be detrimental (Noh et al., 2006). An imbalance between fatty acid uptake and oxidation in aged hearts leads to accumulation of medium-chain acyl-carnitines (Koves et al., 2008) and long-chain fatty acids that are incorporated in triglycerides, phospholipids, and other lipid subspecies, such as diacylglycerols and ceramides. Higher diacylglycerols and ceramides content has been associated with cardiac lipotoxicity, which has been observed in the elderly (Drosatos and Schulze, 2013), as well as in mouse models of cardiac aging along with lower cardiac FAO and higher serum non-esterified fatty acids (Kates et al., 2003; Rodriguez-Calvo et al., 2007; Wang et al., 2015).
Higher cardiac lipid content in aging may be sustained by increased expression of cardiac CD36, which mediates fatty acid transport (Koonen et al., 2007). In accordance, aged CD36-deficient mice have lower levels of intramyocardial lipids, higher mitochondria-derived ATP synthesis, and improved cardiac function compared with aged wild-type mice. While CD36 upregulation and fatty acid uptake seem to be detrimental for cardiac aging, no difference was detected in fasting-induced cardiac lipoprotein lipase activity of aged rats compared with young animals (Bergo et al., 1997). This indicates continuous release of lipoprotein-derived fatty acids in aged hearts, which in combination with higher CD36 expression, explains the increased cardiac lipid content in aging. However, neither inhibition nor activation of lipoprotein lipase offers significant therapeutic potential. Nevertheless, both deletion (Noh et al., 2006) and constitutive activation of lipoprotein lipase (Yagyu et al., 2003) result in cardiomyopathy, due to energetic failure and increased ceramide content (Park et al., 2008), Protein Kinase C signaling activation, and β-adrenergic receptor desensitization (Drosatos et al., 2011).
In humans, age-dependent decline in myocardial fatty acid utilization and ATP production has been observed (Kates et al., 2003). This finding correlates with lower expression of cardiac peroxisome proliferator-activated receptors (PPAR)α (Iemitsu et al., 2002), which is a central transcriptional regulator of proteins that are involved in cardiac energy metabolism (Pol et al., 2015). However, activation of PPARα does not seem to be a desirable intervention, due to similarities of constitutive cardiomyocyte-specific expression of PPARα with cardiomyopathy in diabetes (Finck et al., 2002). On the other hand, cardiomyocyte PPARγ constitutive expression is also toxic for the heart (Son et al., 2007), unless PPARα is inhibited (Son et al., 2010). Thus, as aged hearts have lower PPARα expression (Iemitsu et al., 2002); activation of PPARγ emerges as a potential therapeutic direction that warrants further investigation in models of cardiac aging. Thus the combination of increased lipid uptake and reduced lipid oxidation during cardiac aging may account for the observed cardiac lipotoxicity and associated complications.
Carbohydrate Oxidative Metabolism in Aged Heart
Aged hearts have higher glycolysis and glucose catabolism at the expense of FAO (Lakatta and Yin, 1982). It is also noted that during aging, PDC phosphorylation by PDK is lower, which favors mitochondrial pyruvate oxidation (Moreau et al., 2004). Another study has also reported lower PDK4 expression in aged hearts compared with young hearts (Hyyti et al., 2010). The downregulation of PDK4 may be accounted for by the suppression of the expression of cardiac PPARα that has been reported in aging-associated cardiomyopathy (Iemitsu et al., 2002; Pol et al., 2015). Insulin resistance constitutes another layer of control of glucose utilization that pertains to glucose uptake by the tissues. In elderly, insulin resistance is reckoned as an independent risk factor for coronary heart disease (Butler et al., 2006). It is observed that IGF-1 deficiency enhances resistance of cardiomyocytes against aging (Li et al., 2008). Also, suppression of phosphoinositide 3-kinase (PI3K) weakens senescence and inflammatory markers expression and decreases in accumulation of the aging pigment and lipofuscin (Inuzuka et al., 2009). Thus, shift from FAO to glucose oxidation may constitute a compensatory action of cardiomyocytes aiming to balance the energetic deficiency caused by suppression of FAO. However, the increased glucose utilization in aged hearts and the alleviating effects of insulin signaling inhibition suggest that increased glucose utilization contributes in aging-related cardiomyopathy and may constitute a therapeutic target.
Oxidation of Ketone Bodies in Aged Heart
Recent studies have suggested ketone bodies as an efficient alternative fuel for failing hearts (Takahara et al., 2021), which overall energy production without interfering with fatty acid or glucose catabolism (Ho et al., 2019). In light of the association of increased glucose catabolism with cardiac aging, ketone bodies oxidation emerges as a potential resource that may help alleviate aging-related cardiovascular complication. Adaptation from glycolysis to ketone oxidation is delayed in aged rats, which take longer time than young animals to produce high levels of circulating ketone bodies either when fed on ketogenic diet or following fasting (Okuda et al., 1987; Hernandez et al., 2018).
Ketone body production is increased by approximately 2-fold between 3 and 30 months of age in mice (Robinson and Williamson, 1980). Cardiac SCOT, which is a mitochondrial enzyme involved in the extrahepatic metabolism of ketone bodies, shows increased activity in aged rats due to higher nitro-hydroxyl modification of tryptophan 372 that is proximal to the active site (Rebrin et al., 2007). The beneficial metabolic effect of ketone bodies may rely on their antioxidant properties that pertain to the increased ratio between reduced and oxidized glutathione (Veech et al., 2001; Cahill and Veech, 2003) or their stimulatory effect on succinate oxidation (Balietti et al., 2009). The anti-oxidant effect of ketone bodies, which has been demonstrated by various studies (Mallet and Sun, 2003; Squires et al., 2003), may account for their protective effect in aged hearts as oxidative stress is one of the main manifestations of cardiac aging. The anti-oxidant effect of ketone bodies may also account for the improvements in mitochondrial repair mechanisms (Thai et al., 2019) or in mitophagy flux (Thai et al., 2019) in the aged hearts.
Nutritional interventions with cyclic ketogenic diet in old mice improve heart rate, fractional shortening, left aortic valve pressure gradient, and ventricular mass (Newman et al., 2017). At the same time, ketone bodies incur anti-oxidative and anti-inflammatory effects and counteract age-related cardiac remodeling (Shimazu et al., 2013; Puchalska and Crawford, 2017). Despite the beneficial metabolic effects of ketone bodies, it has been reported that they compete with fatty acids and inhibit their oxidation in diabetic hearts (Hasselbaink et al., 2003; Stanley et al., 2003), which results in lipid accumulation. Studies with isolated cardiomyocytes showed that chronic exposure to βOHB induces insulin resistance and suppresses glucose uptake and oxidation (Pelletier et al., 2007). Taking into account the association of glucose oxidation with cardiotoxicity in aging, ketone bodies hold promise for therapeutic interventions that aim to alleviate cardiomyopathy in the elderly.
Mitochondrial Function in Cardiac Aging
Due to the high-energy demands of the heart, cardiomyocytes are rich in mitochondria, which account for ~35% of total cellular volume. Therefore, the heart is highly vulnerable to mitochondrial anomalies. There are two types of cardiac mitochondria with varying activities: the subsarcolemmal mitochondria (SSM) and the interfibrillar mitochondria (IFM) that are located between the myofibrils (Palmer et al., 1977). Aging primarily decreases content and function of IFM, associated with lower activity of mitochondrial respiratory chain complexes III and IV, impaired oxidative phosphorylation, and increased production of reactive oxygen species (ROS) that leads to injury (Fannin et al., 1999; Judge et al., 2005). Studies in humans and animals have reported that aged hearts have larger mitochondria with disrupted structure and inner membrane cristae (Sachs et al., 1977; Dai and Rabinovitch, 2009). Other studies have also reported lower content and activity of cytochrome oxidase in aged human hearts. This event has deleterious effect on cardiac energy production and exerts cytotoxicity (Fujioka et al., 2011).
Cardiac mitochondria of the elderly are more sensitive to lipid peroxidation and dysfunction when exposed to exogenous iron and H2O2 (Sawada and Carlson, 1987; Cusack et al., 1991). Aged mice have significantly lower expression of myocardial mitochondrial deacetylase sirtuin (SIRT)3, which counteracts oxidative stress. In young animals, nuclear factor erythroid-2 related factor (NRF-2), a highly conserved redox-sensitive transcription factor, is activated by ROS production and increases the expression of several antioxidant genes. However, in aging, ROS-dependent activation of NRF-2 is defective, which aggravates the deleterious effects of ROS (Warabi et al., 2007; Ungvari et al., 2011). Manganese superoxide dismutase, which transforms toxic superoxide to hydrogen peroxide and diatomic oxygen, thus preventing accumulation of mitochondrial ROS, is found to be reduced in aged hearts (Li et al., 2018). Along these lines, supplementation of polyunsaturated fatty acid-containing diet with the anti-oxidant coenzyme Q10 in 24-month old rats increased catalase activity, reduced H2O2 generation, improved cytochrome oxidase activity in cardiac mitochondria, and extended life span (Ochoa et al., 2005; Quiles et al., 2010).
In addition, mitochondrial protein carbonylation is increased with aging, which further aggravates mitochondrial oxidative damage (Dai et al., 2012). Mitochondrial DNA (mtDNA), which lacks protective histones, is particularly vulnerable to oxidative damage leading to point mutations and deletions, inflammation, apoptosis, telomere shortening, necrosis, and immunological dysfunction (Larsson, 2010; Steenman and Lande, 2017).
Besides oxidative stress, mitochondrial biogenesis is also compromised during cardiac aging. PGC-1α, which stimulates mitochondrial biogenesis, serves as a transcriptional coactivator of nuclear receptors, like PPARs, Estrogen-related receptor alpha (ERRα), nuclear factor erythroid-2 related factor (NRF)-1 and NRF-2 (Sanchis-Gomar et al., 2014; Dorn et al., 2015), and regulator of cardiac fatty acid utilization (Arany et al., 2005; Rowe et al., 2010). PGC-1α protein levels are lower in aged mice, compared to young mice (Conley et al., 2007; Vina et al., 2009) similar to what is observed in humans with heart failure (Sihag et al., 2009). PGC-1α knockout in young mice mimics age-related impairments in mitochondrial gene expression. On the other hand, overt activation of PGC-1α accelerates cardiac aging and reduces life span of old wild type mice because of mitochondrial damage and ROS accumulation (Zhu et al., 2019), indicating a delicate balance between PGC1α activation and inhibition for cardiac homeostasis. Indeed, moderate PGC-1α activation inhibits age-related cardiac remodeling changes, such as apoptosis and collagen accumulation, and increases the expression of genes involved in myocardial contractility, metabolism, biogenesis, dynamics, and calcium handling (Whitehead et al., 2018). In accordance with the genetic interventions, mild activators of SIRT1 and PGC-1α, such as resveratrol, incur beneficial effects in mitochondria of senescent HL-1 cardiomyocytes (Ren et al., 2017).
Mitochondrial function is regulated by cardiolipin, which is a main phospholipid of the inner mitochondrial membrane. Cardiolipin preserves the morphology and architecture of the mitochondrial membrane and regulates the activity of mitochondrial enzymes and proteins (Paradies et al., 2019). Some studies have attributed mitochondrial complications during aging to lower cardiolipin content that alters fluidity of the inner mitochondrial membrane. During oxidative stress, cardiolipin undergoes oxidation that disrupts the electron transport chain, leading to mitochondrial dysfunction, cytochrome c release, and cellular apoptosis (Paradies et al., 1998; Ott et al., 2002; Chicco and Sparagna, 2007).
Mitochondrial quality in cardiomyocytes is maintained through a balance between mitophagy and biogenesis. The mitochondrial defects that are observed during cardiac aging are further aggravated by impaired fission, fusion, autophagy, and lysosomal degradation. To correct flaws in mitochondrial function, there is a quality control process that includes biogenesis of new mitochondria and degradation or recycling of the old ones. Accumulation of dysfunctional mitochondria due to oxidative stress, mtDNA mutations, lowering of mitochondrial membrane potential, and defective ATP synthesis stimulate cellular apoptosis and premature cardiac aging. The frequency of mtDNA mutations in older mice is 1,000-fold higher than that for nuclear genes. Accordingly, human cardiac mtDNA deletions begin at the age of 40 years and accumulate gradually with age (Corral-Debrinski et al., 1992; Khaidakov et al., 2003).
Thus, reductions in mitochondrial number along with accumulation of defective mitochondria that constitute a source of ROS are major contributors of the pathophysiology of cardiac aging.
Autophagy in Cardiac Aging
Autophagy is a highly conserved homeostatic mechanism in cells. It includes micro-autophagy, macro-autophagy, and chaperone-mediated autophagy and is regulated by a series of autophagy related genes (ATG). It is involved in degradation and recycling of cellular components, removal of misfolded proteins and damaged organelles, and internal “recycling” of nutrients (Rubinsztein et al., 2011). In cells with low proliferative capacity, like cardiomyocytes, accumulation of lethal proteins, and dysfunctional organelles is a critical event that impairs cellular homeostasis. As age advances the rate of autophagy and mitophagy decrease in heart as well as in other tissues (Hoshino et al., 2013; Xu et al., 2016). The decline in autophagy has a significant impact on the aging-associated accumulation of defective mitochondria and consequent ROS generation in cariomyocytes leading to mitochondrial dysfunction, cardiac aging, and myocardial injury (Mammucari and Rizzuto, 2010; Rezzani et al., 2012). Accordingly, autophagy is a vital mechanism for maintenance of homeostasis and adaptation to stress that the heart undergoes throughout the aging process (Levine and Kroemer, 2008). Lower autophagy that occurs during cardiac aging, results in the accumulation of protein aggregates, oxidized and damaged proteins, and lipofuscin (Dai et al., 2010). Lipofuscin is rich in iron and therefore, it causes lysosomal rupture, oxidative stress, and further mitochondrial damage (Brunk and Terman, 2002). Mouse models of diet-induced obesity with impaired autophagy have compromised myocardial geometry and function (Xu et al., 2013), which indicates that autophagy also alleviates cardiac lipotoxicity.
Protein carbonylation and ubiquitination in aged hearts along with reduced protein turnover rates (Basisty et al., 2018) compromise cardiac proteasome function that impairs protein quality control and leads to accumulation of damaged and misfolded proteins. It is also noted alteration in the expression of heat shock proteins (HSP), which function as molecular chaperones facilitating protein folding and targeting improperly folded proteins to degradative pathways are reduced in aged cardiomyocytes (Bodyak et al., 2002). Higher levels of ROS in aged cardiomyocytes stimulate formation of lipofuscin aggregates, consisting of lipids and aggregated liposomal proteins in lysosomes and which distort autophagy by preventing the fusion of autophagosomes with lysosomes leads to accumulation of aberrant proteins and dysfunctional mitochondria, less effective stress response, and functional loss in cardiomyocytes (Taneike et al., 2010).
Stimulation of autophagy improves cardiac function and prolongs the lifespan in many organisms (Miyamoto, 2019). Selective elimination of damaged mitochondria by autophagy (mitophagy) is an essential component of this mechanism. Rapamycin, which inhibits mTOR and induces autophagy, improves cardiac function and extends life span in C57BL/6 mice, which is also accompanied by increased mitochondrial biogenesis and fatty acid catabolism (Dai et al., 2014; Zhang et al., 2014; Quarles et al., 2020). Another intervention that activated autophagy, which rely on overexpression of ATG5, extended life span in mice. Accordingly, ATG5 deficiency in mouse hearts induces age-associated cardiomyopathy (Taneike et al., 2010; Pyo et al., 2013).
Beclin 1 regulates the initial stage of autophagy in mammalian cells by leading to the formation of a double-membrane structure that engulfs cytoplasmic material to form autophagosomes. Bcl-2 is inhibiting activity of beclin 1 by directly binding to it (Qu et al., 2003; Pattingre et al., 2005). Disruption of the Beclin1-Bcl2 complex stimulates autophagy and inhibits age-induced cardiac fibrosis, hypertrophy, apoptotic cell death, and delays cardiac aging (Fernandez et al., 2018).
Parkin is an E3 ligase that mediates selective identification and removal of damaged mitochondria by autophagy. Parkin-deficient mice accumulate unusual mitochondria in the heart as they age, while parkin transgenic mice display increased mitophagy and are resistant to cardiac aging (Hoshino et al., 2013; Kubli et al., 2013).
The mitochondrial membrane depolarization-dependent PTEN-induced putative kinase (PINK)1/Parkin pathway is involved in tagging defective mitochondria, which is vital for maintaining cardiac function during aging (Nguyen et al., 2016). Autophagy is controlled by various metabolism-related pathways, including the IGF-1/Akt pathway (Ock et al., 2016), the mammalian target of rapamycin (mTORC1) pathway (Harrison et al., 2009), the AMP-activated protein kinase (AMPK) pathway (Kim et al., 2011), Forkhead box O (FoxO), the transcription factor EB (TFEB) pathway (Fullgrabe et al., 2016), ROS (Shirakabe et al., 2016), and sirtuins (Alcendor et al., 2007).
Thus, aging-related cardiomyopathy is associated with lower autophagic flux. Hence, autophagy is a promising therapeutic target for cardiac aging.
Metabolic Deficiency and Inflammation in Cardiac Aging
A close link between cellular oxidative stress and inflammation exists and contributes in the cardiac aging process (De La Fuente, 2002). Inflammation is stimulated by accumulation of senescent cardiomyocytes that leads to senescence-associated secretory phenotype (SASP). GDF15, Tgfb2, and Edn3 are nontypical SASP that are secreted by cardiomyocytes. Chronic stimulation of the immune system attenuates immune response, which is typical for inflammation-associated aging (“inflammaging”; Douziech et al., 2002) that is controlled by NF-κB (Salminen et al., 2008a). Inhibition of aging-related NF-κB activation by the longevity gene, SIRT1 delays aging (Salminen et al., 2008b; Cevenini et al., 2013). Circulating levels of activin, a senescence-associated secreted protein has been correlated with age-related heart failure (Li et al., 2020). Thus, defects in the metabolic machinery of aged cardiomyocytes exert pro-inflammatory effects that compromise cardiac function further.
Epilogue
Heart is an organ with high energetic demands that are achieved through oxidation of various substrates. Utilization of fatty acids, which is the major substrate for energy production, is reduced during cardiac aging at the expense of higher carbohydrate oxidation. This leads to accumulation of toxic lipids in the heart. These events are associated with mitochondrial dysfunction and eventual accumulation of ROS that further aggravate cardiac dysfunction. The higher inflammatory status and impaired autophagy compromise reparative processes that could further worsen aging-related cardiomyopathy (Figure 2).
Figure 2.
Metabolic and mitochondrial complications in cardiac aging. Figure was produced using Servier medical art (http://www.servier.com).
The variety of metabolic pathways that are affected during cardiac aging suggests potential novel therapeutic interventions. Particularly, interventions that aim to negate aging-induced defects in mitochondria and intermediate metabolism are warranted. Based on clinical observations and preclinical interventions, treatments that will stimulate oxidation of fatty acids and ketone bodies, while they will simultaneously limit glucose catabolism, maintain normal autophagic flux, and limit mitochondrial ROS accumulation hold significant therapeutic potential. Thus, additional studies aiming to elucidate metabolic changes that occur during the progression of cardiac aging are warranted, in order to identify novel therapeutic avenues that may eventually translate to treatments of aging-related cardiovascular complications in humans.
Author Contributions
TS reviewed the literature and wrote the manuscript. KD reviewed the literature and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
- Alcendor R. R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., et al. (2007). Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100, 1512–1521. 10.1161/01.RES.0000267723.65696.4a, PMID: [DOI] [PubMed] [Google Scholar]
- Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., et al. (2005). Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271. 10.1016/j.cmet.2005.03.002, PMID: [DOI] [PubMed] [Google Scholar]
- Balietti M., Fattoretti P., Giorgetti B., Casoli T., Di Stefano G., Solazzi M., et al. (2009). A ketogenic diet increases succinic dehydrogenase activity in aging cardiomyocytes. Ann. N. Y. Acad. Sci. 1171, 377–384. 10.1111/j.1749-6632.2009.04704.x, PMID: [DOI] [PubMed] [Google Scholar]
- Basisty N., Meyer J. G., Schilling B. (2018). Protein turnover in aging and longevity. Proteomics 18:e1700108. 10.1002/pmic.201700108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo M., Olivecrona G., Olivecrona T. (1997). Regulation of adipose tissue lipoprotein lipase in young and old rats. Int. J. Obes. Relat. Metab. Disord. 21, 980–986. 10.1038/sj.ijo.0800506, PMID: [DOI] [PubMed] [Google Scholar]
- Bharadwaj K. G., Hiyama Y., Hu Y., Huggins L. A., Ramakrishnan R., Abumrad N. A., et al. (2010). Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J. Biol. Chem. 285, 37976–37986. 10.1074/jbc.M110.174458, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N., Kang P. M., Hiromura M., Sulijoadikusumo I., Horikoshi N., Khrapko K., et al. (2002). Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res. 30, 3788–3794. 10.1093/nar/gkf497, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk U. T., Terman A. (2002). The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 269, 1996–2002. 10.1046/j.1432-1033.2002.02869.x, PMID: [DOI] [PubMed] [Google Scholar]
- Butler J., Rodondi N., Zhu Y., Figaro K., Fazio S., Vaughan D. E., et al. (2006). Metabolic syndrome and the risk of cardiovascular disease in older adults. J. Am. Coll. Cardiol. 47, 1595–1602. 10.1016/j.jacc.2005.12.046, PMID: [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr., Veech R. L. (2003). Ketoacids? Good medicine? Trans. Am. Clin. Climatol. Assoc. 114, 149–161 discussion 162-143. PMID: [PMC free article] [PubMed] [Google Scholar]
- Cevenini E., Monti D., Franceschi C. (2013). Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 16, 14–20. 10.1097/MCO.0b013e32835ada13, PMID: [DOI] [PubMed] [Google Scholar]
- Chicco A. J., Sparagna G. C. (2007). Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Phys. Cell Phys. 292, C33–C44. 10.1152/ajpcell.00243.2006, PMID: [DOI] [PubMed] [Google Scholar]
- Coburn C. T., Knapp F. F., Jr., Febbraio M., Beets A. L., Silverstein R. L., Abumrad N. A. (2000). Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529. 10.1074/jbc.M003826200, PMID: [DOI] [PubMed] [Google Scholar]
- Conley K. E., Marcinek D. J., Villarin J. (2007). Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care 10, 688–692. 10.1097/MCO.0b013e3282f0dbfb, PMID: [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Shoffner J. M., Lott M. T., Wallace D. C. (1992). Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat. Res. 275, 169–180. 10.1016/0921-8734(92)90021-g, PMID: [DOI] [PubMed] [Google Scholar]
- Cotter D. G., Schugar R. C., Crawford P. A. (2013). Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 304, H1060–H1076. 10.1152/ajpheart.00646.2012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack B. J., Mushlin P. S., Andrejuk T., Voulelis L. D., Olson R. D. (1991). Aging alters the force-frequency relationship and toxicity of oxidative stress in rabbit heart. Life Sci. 48, 1769–1777. 10.1016/0024-3205(91)90215-W, PMID: [DOI] [PubMed] [Google Scholar]
- Dai D. F., Chen T., Johnson S. C., Szeto H., Rabinovitch P. S. (2012). Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 16, 1492–1526. 10.1089/ars.2011.4179, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. F., Chen T., Wanagat J., Laflamme M., Marcinek D. J., Emond M. J., et al. (2010). Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544. 10.1111/j.1474-9726.2010.00581.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. F., Karunadharma P. P., Chiao Y. A., Basisty N., Crispin D., Hsieh E. J., et al. (2014). Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539. 10.1111/acel.12203, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. F., Rabinovitch P. S. (2009). Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 19, 213–220. 10.1016/j.tcm.2009.12.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente M. (2002). Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 56(Suppl. 3), S5–S8. 10.1038/sj.ejcn.1601476, PMID: [DOI] [PubMed] [Google Scholar]
- Dorn G. W., 2nd, Vega R. B., Kelly D. P. (2015). Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29, 1981–1991. 10.1101/gad.269894.115, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douziech N., Seres I., Larbi A., Szikszay E., Roy P. M., Arcand M., et al. (2002). Modulation of human lymphocyte proliferative response with aging. Exp. Gerontol. 37, 369–387. 10.1016/S0531-5565(01)00204-2, PMID: [DOI] [PubMed] [Google Scholar]
- Drosatos K., Bharadwaj K. G., Lymperopoulos A., Ikeda S., Khan R., Hu Y., et al. (2011). Cardiomyocyte lipids impair beta-adrenergic receptor function via PKC activation. Am. J. Physiol. Endocrinol. Metab. 300, E489–E499. 10.1152/ajpendo.00569.2010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosatos K., Schulze P. C. (2013). Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 10, 109–121. 10.1007/s11897-013-0133-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannin S. W., Lesnefsky E. J., Slabe T. J., Hassan M. O., Hoppel C. L. (1999). Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 372, 399–407. 10.1006/abbi.1999.1508, PMID: [DOI] [PubMed] [Google Scholar]
- Fernandez A. F., Sebti S., Wei Y., Zou Z., Shi M., Mcmillan K. L., et al. (2018). Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558, 136–140. 10.1038/s41586-018-0162-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., et al. (2002). The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109, 121–130. 10.1172/JCI0214080, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H., Moghaddas S., Murdock D. G., Lesnefsky E. J., Tandler B., Hoppel C. L. (2011). Decreased cytochrome c oxidase subunit VIIa in aged rat heart mitochondria: immunocytochemistry. Anat. Rec. 294, 1825–1833. 10.1002/ar.21486, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullgrabe J., Ghislat G., Cho D. H., Rubinsztein D. C. (2016). Transcriptional regulation of mammalian autophagy at a glance. J. Cell Sci. 129, 3059–3066. 10.1242/jcs.188920, PMID: [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. 10.1038/nature08221, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbaink D. M., Glatz J. F., Luiken J. J., Roemen T. H., Van Der Vusse G. J. (2003). Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem. J. 371, 753–760. 10.1042/bj20021617, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A. R., Hernandez C. M., Campos K. T., Truckenbrod L. M., Sakarya Y., Mcquail J. A., et al. (2018). The antiepileptic ketogenic diet alters hippocampal transporter levels and reduces adiposity in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 73, 450–458. 10.1093/gerona/glx193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. L., Zhang L., Wagg C., Al Batran R., Gopal K., Levasseur J., et al. (2019). Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 115, 1606–1616. 10.1093/cvr/cvz045, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppel C. L., Kerner J., Turkaly P., Turkaly J., Tandler B. (1998). The malonyl-CoA-sensitive form of carnitine palmitoyltransferase is not localized exclusively in the outer membrane of rat liver mitochondria. J. Biol. Chem. 273, 23495–23503. 10.1074/jbc.273.36.23495, PMID: [DOI] [PubMed] [Google Scholar]
- Hoshino A., Mita Y., Okawa Y., Ariyoshi M., Iwai-Kanai E., Ueyama T., et al. (2013). Cytosolic p53 inhibits parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4:2308. 10.1038/ncomms3308, PMID: [DOI] [PubMed] [Google Scholar]
- Hyyti O. M., Ledee D., Ning X. H., Ge M., Portman M. A. (2010). Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am. J. Physiol. Heart Circ. Physiol. 299, H868–H875. 10.1152/ajpheart.00931.2009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemitsu M., Miyauchi T., Maeda S., Tanabe T., Takanashi M., Irukayama-Tomobe Y., et al. (2002). Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am. J. Physiol. Heart Circ. Physiol. 283, H1750–H1760. 10.1152/ajpheart.01051.2001, PMID: [DOI] [PubMed] [Google Scholar]
- Inuzuka Y., Okuda J., Kawashima T., Kato T., Niizuma S., Tamaki Y., et al. (2009). Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120, 1695–1703. 10.1161/CIRCULATIONAHA.109.871137, PMID: [DOI] [PubMed] [Google Scholar]
- Judge S., Jang Y. M., Smith A., Hagen T., Leeuwenburgh C. (2005). Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 19, 419–421. 10.1096/fj.04-2622fje, PMID: [DOI] [PubMed] [Google Scholar]
- Karwi Q. G., Uddin G. M., Ho K. L., Lopaschuk G. D. (2018). Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5:68. 10.3389/fcvm.2018.00068, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates A. M., Herrero P., Dence C., Soto P., Srinivasan M., Delano D. G., et al. (2003). Impact of aging on substrate metabolism by the human heart. J. Am. Coll. Cardiol. 41, 293–299. 10.1016/s0735-1097(02)02714-6, PMID: [DOI] [PubMed] [Google Scholar]
- Khaidakov M., Heflich R. H., Manjanatha M. G., Myers M. B., Aidoo A. (2003). Accumulation of point mutations in mitochondrial DNA of aging mice. Mutat. Res. 526, 1–7. 10.1016/s0027-5107(03)00010-1, PMID: [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141. 10.1038/ncb2152, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonen D. P., Febbraio M., Bonnet S., Nagendran J., Young M. E., Michelakis E. D., et al. (2007). CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation 116, 2139–2147. 10.1161/CIRCULATIONAHA.107.712901, PMID: [DOI] [PubMed] [Google Scholar]
- Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., et al. (2008). Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56. 10.1016/j.cmet.2007.10.013, PMID: [DOI] [PubMed] [Google Scholar]
- Kubli D. A., Quinsay M. N., Gustafsson A. B. (2013). Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun. Integr. Biol. 6:e24511. 10.4161/cib.24511, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E. G., Yin F. C. (1982). Myocardial aging: functional alterations and related cellular mechanisms. Am. J. Phys. 242, H927–H941. 10.1152/ajpheart.1982.242.6.H927, PMID: [DOI] [PubMed] [Google Scholar]
- Larsson N. G. (2010). Somatic mitochondrial DNA mutations in mammalian aging. Annu. Rev. Biochem. 79, 683–706. 10.1146/annurev-biochem-060408-093701, PMID: [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. (2008). Autophagy in the pathogenesis of disease. Cell 132, 27–42. 10.1016/j.cell.2007.12.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ceylan-Isik A. F., Li J., Ren J. (2008). Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 11, 725–733. 10.1089/rej.2008.0717, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Hastings M. H., Rhee J., Trager L. E., Roh J. D., Rosenzweig A. (2020). Targeting age-related pathways in heart failure. Circ. Res. 126, 533–551. 10.1161/CIRCRESAHA.119.315889, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ma Y., Song L., Yu L., Zhang L., Zhang Y., et al. (2018). SIRT3 deficiency exacerbates p53/Parkin mediated mitophagy inhibition and promotes mitochondrial dysfunction: Implication for aged hearts. Int. J. Mol. Med. 41, 3517–3526. 10.3892/ijmm.2018.3555 [DOI] [PubMed] [Google Scholar]
- Lopaschuk G. D. (2017). Metabolic modulators in heart disease: past, present, and future. Can. J. Cardiol. 33, 838–849. 10.1016/j.cjca.2016.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- Lopaschuk G. D., Ussher J. R., Folmes C. D., Jaswal J. S., Stanley W. C. (2010). Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258. 10.1152/physrev.00015.2009, PMID: [DOI] [PubMed] [Google Scholar]
- Ma Y., Li J. (2015). Metabolic shifts during aging and pathology. Compr. Physiol. 5, 667–686. 10.1002/cphy.c140041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodzadeh S., Eder S., Nordmeyer J., Ehler E., Huber O., Martus P., et al. (2006). Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 20, 926–934. 10.1096/fj.05-5148com, PMID: [DOI] [PubMed] [Google Scholar]
- Mallet R. T., Sun J. (2003). Antioxidant properties of myocardial fuels. Mol. Cell. Biochem. 253, 103–111. 10.1023/A:1026009519783, PMID: [DOI] [PubMed] [Google Scholar]
- Mammucari C., Rizzuto R. (2010). Signaling pathways in mitochondrial dysfunction and aging. Mech. Ageing Dev. 131, 536–543. 10.1016/j.mad.2010.07.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz A. A., Cheng S. (2016). Sex differences in cardiovascular ageing. Heart 102, 825–831. 10.1136/heartjnl-2015-308769, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S. (2019). Autophagy and cardiac aging. Cell Death Differ. 26, 653–664. 10.1038/s41418-019-0286-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R., Heath S. H., Doneanu C. E., Harris R. A., Hagen T. M. (2004). Age-related compensatory activation of pyruvate dehydrogenase complex in rat heart. Biochem. Biophys. Res. Commun. 325, 48–58. 10.1016/j.bbrc.2004.10.011, PMID: [DOI] [PubMed] [Google Scholar]
- Newman J. C., Covarrubias A. J., Zhao M., Yu X., Gut P., Ng C. P., et al. (2017). Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547.e548–557.e548. 10.1016/j.cmet.2017.08.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. N., Padman B. S., Lazarou M. (2016). Deciphering the molecular signals of PINK1/parkin mitophagy. Trends Cell Biol. 26, 733–744. 10.1016/j.tcb.2016.05.008, PMID: [DOI] [PubMed] [Google Scholar]
- Noh H. L., Okajima K., Molkentin J. D., Homma S., Goldberg I. J. (2006). Acute lipoprotein lipase deletion in adult mice leads to dyslipidemia and cardiac dysfunction. Am. J. Physiol. Endocrinol. Metab. 291, E755–E760. 10.1152/ajpendo.00111.2006, PMID: [DOI] [PubMed] [Google Scholar]
- Ochoa J. J., Quiles J. L., Huertas J. R., Mataix J. (2005). Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 60, 970–975. 10.1093/gerona/60.8.970, PMID: [DOI] [PubMed] [Google Scholar]
- Ock S., Lee W. S., Ahn J., Kim H. M., Kang H., Kim H. S., et al. (2016). Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology 157, 336–345. 10.1210/en.2015-1709, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y., Kawai K., Yamashita K. (1987). Age-related change in ketone body metabolism: diminished glucagon effect on ketogenesis in adult rats. Endocrinology 120, 2152–2157. 10.1210/endo-120-5-2152, PMID: [DOI] [PubMed] [Google Scholar]
- Ott M., Robertson J. D., Gogvadze V., Zhivotovsky B., Orrenius S. (2002). Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U. S. A. 99, 1259–1263. 10.1073/pnas.241655498, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. W., Tandler B., Hoppel C. L. (1977). Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 252, 8731–8739. 10.1016/S0021-9258(19)75283-1, PMID: [DOI] [PubMed] [Google Scholar]
- Paradies G., Paradies V., Ruggiero F. M., Petrosillo G. (2019). Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cell 8:728. 10.3390/cells8070728, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G., Ruggiero F. M., Petrosillo G., Quagliariello E. (1998). Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett. 424, 155–158. 10.1016/S0014-5793(98)00161-6, PMID: [DOI] [PubMed] [Google Scholar]
- Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., et al. (2008). Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112. 10.1194/jlr.M800147-JLR200, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S., Tassa A., Qu X., Garuti R., Liang X. H., Mizushima N., et al. (2005). Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, 927–939. 10.1016/j.cell.2005.07.002, PMID: [DOI] [PubMed] [Google Scholar]
- Pelletier A., Tardif A., Gingras M. H., Chiasson J. L., Coderre L. (2007). Chronic exposure to ketone bodies impairs glucose uptake in adult cardiomyocytes in response to insulin but not vanadate: the role of PI3-K. Mol. Cell. Biochem. 296, 97–108. 10.1007/s11010-006-9303-7, PMID: [DOI] [PubMed] [Google Scholar]
- Pol C. J., Lieu M., Drosatos K. (2015). PPARs: protectors or opponents of myocardial function? PPAR Res. 2015:835985. 10.1155/2015/835985, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalska P., Crawford P. A. (2017). Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 25, 262–284. 10.1016/j.cmet.2016.12.022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo J. O., Yoo S. M., Ahn H. H., Nah J., Hong S. H., Kam T. I., et al. (2013). Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 4:2300. 10.1038/ncomms3300, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., et al. (2003). Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820. 10.1172/JCI20039, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles E., Basisty N., Chiao Y. A., Merrihew G., Gu H., Sweetwyne M. T., et al. (2020). Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 19:e13086. 10.1111/acel.13086, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles J. L., Pamplona R., Ramirez-Tortosa M. C., Naudi A., Portero-Otin M., Araujo-Nepomuceno E., et al. (2010). Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart. Mech. Ageing Dev. 131, 38–47. 10.1016/j.mad.2009.11.004, PMID: [DOI] [PubMed] [Google Scholar]
- Ramos-Roman M. A., Sweetman L., Valdez M. J., Parks E. J. (2012). Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism 61, 202–212. 10.1016/j.metabol.2011.06.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I., Bregere C., Kamzalov S., Gallaher T. K., Sohal R. S. (2007). Nitration of tryptophan 372 in succinyl-CoA:3-ketoacid CoA transferase during aging in rat heart mitochondria. Biochemistry 46, 10130–10144. 10.1021/bi7001482, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Chen L., Xie J., Zhang Z., Dong G., Liang J., et al. (2017). Resveratrol ameliorates mitochondrial elongation via Drp1/parkin/PINK1 signaling in senescent-Like Cardiomyocytes. Oxidative Med. Cell. Longev. 2017:4175353. 10.1155/2017/4175353, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzani R., Stacchiotti A., Rodella L. F. (2012). Morphological and biochemical studies on aging and autophagy. Ageing Res. Rev. 11, 10–31. 10.1016/j.arr.2011.09.001, PMID: [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. (1980). Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 60, 143–187. 10.1152/physrev.1980.60.1.143, PMID: [DOI] [PubMed] [Google Scholar]
- Rodriguez-Calvo R., Serrano L., Barroso E., Coll T., Palomer X., Camins A., et al. (2007). Peroxisome proliferator-activated receptor alpha down-regulation is associated with enhanced ceramide levels in age-associated cardiac hypertrophy. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1326–1336. 10.1093/gerona/62.12.1326, PMID: [DOI] [PubMed] [Google Scholar]
- Rowe G. C., Jiang A., Arany Z. (2010). PGC-1 coactivators in cardiac development and disease. Circ. Res. 107, 825–838. 10.1161/CIRCRESAHA.110.223818, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C., Marino G., Kroemer G. (2011). Autophagy and aging. Cell 146, 682–695. 10.1016/j.cell.2011.07.030, PMID: [DOI] [PubMed] [Google Scholar]
- Sachs H. G., Colgan J. A., Lazarus M. L. (1977). Ultrastructure of the aging myocardium: a morphometric approach. Am. J. Anat. 150, 63–71. 10.1002/aja.1001500105, PMID: [DOI] [PubMed] [Google Scholar]
- Salminen A., Huuskonen J., Ojala J., Kauppinen A., Kaarniranta K., Suuronen T. (2008a). Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 7, 83–105. 10.1016/j.arr.2007.09.002, PMID: [DOI] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Suuronen T., Kaarniranta K. (2008b). SIRT1 longevity factor suppresses NF-kappaB-driven immune responses: regulation of aging via NF-kappaB acetylation? BioEssays 30, 939–942. 10.1002/bies.20799, PMID: [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F., Garcia-Gimenez J. L., Gomez-Cabrera M. C., Pallardo F. V. (2014). Mitochondrial biogenesis in health and disease. molecular and therapeutic approaches. Curr. Pharm. Des. 20, 5619–5633. 10.2174/1381612820666140306095106, PMID: [DOI] [PubMed] [Google Scholar]
- Sawada M., Carlson J. C. (1987). Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Ageing Dev. 41, 125–137. 10.1016/0047-6374(87)90057-1, PMID: [DOI] [PubMed] [Google Scholar]
- Shimazu T., Hirschey M. D., Newman J., He W., Shirakawa K., Le Moan N., et al. (2013). Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214. 10.1126/science.1227166, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D. K., Sadoshima J. (2016). Aging and autophagy in the heart. Circ. Res. 118, 1563–1576. 10.1161/CIRCRESAHA.116.307474, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag S., Cresci S., Li A. Y., Sucharov C. C., Lehman J. J. (2009). PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J. Mol. Cell. Cardiol. 46, 201–212. 10.1016/j.yjmcc.2008.10.025, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son N. H., Basu D., Samovski D., Pietka T. A., Peche V. S., Willecke F., et al. (2018). Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Invest. 128, 4329–4342. 10.1172/JCI99315, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., et al. (2007). Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 117, 2791–2801. 10.1172/JCI30335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son N. H., Yu S., Tuinei J., Arai K., Hamai H., Homma S., et al. (2010). PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J. Clin. Invest. 120, 3443–3454. 10.1172/JCI40905, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J. E., Sun J., Caffrey J. L., Yoshishige D., Mallet R. T. (2003). Acetoacetate augments beta-adrenergic inotropism of stunned myocardium by an antioxidant mechanism. Am. J. Physiol. Heart Circ. Physiol. 284, H1340–H1347. 10.1152/ajpheart.00473.2002, PMID: [DOI] [PubMed] [Google Scholar]
- Stanley W. C., Meadows S. R., Kivilo K. M., Roth B. A., Lopaschuk G. D. (2003). Beta-hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am. J. Physiol. Heart Circ. Physiol. 285, H1626–H1631. 10.1152/ajpheart.00332.2003, PMID: [DOI] [PubMed] [Google Scholar]
- Steenman M., Lande G. (2017). Cardiac aging and heart disease in humans. Biophys. Rev. 9, 131–137. 10.1007/s12551-017-0255-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara S., Soni S., Maayah Z. H., Ferdaoussi M., Dyck J. R. B. (2021). Ketone therapy for heart failure: current evidence for clinical use. Cardiovasc. Res. cvab068. 10.1093/cvr/cvab068, PMID: [Ebup ahead of print] [DOI] [PubMed]
- Taneike M., Yamaguchi O., Nakai A., Hikoso S., Takeda T., Mizote I., et al. (2010). Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606. 10.4161/auto.6.5.11947, PMID: [DOI] [PubMed] [Google Scholar]
- Thai P. N., Seidlmayer L. K., Miller C., Ferrero M., Dorn G. W., II, Schaefer S., et al. (2019). Mitochondrial quality control in aging and heart failure: influence of ketone bodies and mitofusin-stabilizing peptides. Front. Physiol. 10:382. 10.3389/fphys.2019.00382, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomec R. J., Hoppel C. L. (1975). Carnitine palmitoyltransferase in bovine fetal heart mitochondria. Arch. Biochem. Biophys. 170, 716–723. 10.1016/0003-9861(75)90169-1, PMID: [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Bailey-Downs L., Sosnowska D., Gautam T., Koncz P., Losonczy G., et al. (2011). Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 301, H363–H372. 10.1152/ajpheart.01134.2010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Chance B., Kashiwaya Y., Lardy H. A., Cahill G. F., Jr. (2001). Ketone bodies, potential therapeutic uses. IUBMB Life 51, 241–247. 10.1080/152165401753311780, PMID: [DOI] [PubMed] [Google Scholar]
- Vina J., Gomez-Cabrera M. C., Borras C., Froio T., Sanchis-Gomar F., Martinez-Bello V. E., et al. (2009). Mitochondrial biogenesis in exercise and in ageing. Adv. Drug Deliv. Rev. 61, 1369–1374. 10.1016/j.addr.2009.06.006, PMID: [DOI] [PubMed] [Google Scholar]
- Viscarra J. A., Ortiz R. M. (2013). Cellular mechanisms regulating fuel metabolism in mammals: role of adipose tissue and lipids during prolonged food deprivation. Metabolism 62, 889–897. 10.1016/j.metabol.2012.12.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., West J. A., Murray A. J., Griffin J. L. (2015). Comprehensive metabolic profiling of age-related mitochondrial dysfunction in the high-fat-fed ob/ob mouse heart. J. Proteome Res. 14, 2849–2862. 10.1021/acs.jproteome.5b00128, PMID: [DOI] [PubMed] [Google Scholar]
- Warabi E., Takabe W., Minami T., Inoue K., Itoh K., Yamamoto M., et al. (2007). Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic. Biol. Med. 42, 260–269. 10.1016/j.freeradbiomed.2006.10.043, PMID: [DOI] [PubMed] [Google Scholar]
- Whitehead N., Gill J. F., Brink M., Handschin C. (2018). Moderate modulation of cardiac PGC-1alpha expression partially affects age-associated transcriptional remodeling of the heart. Front. Physiol. 9:242. 10.3389/fphys.2018.00242, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., Spray G. H., Hems R., Williamson D. H. (1971). Metabolic effects of propionate in normal and vitamin B 12-deficient rats. Biochem. J. 124, 501–507. 10.1042/bj1240501, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hua Y., Nair S., Zhang Y., Ren J. (2013). Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J. Mol. Cell Biol. 5, 61–63. 10.1093/jmcb/mjs055, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Pang J., Chen Y., Bucala R., Zhang Y., Ren J. (2016). Macrophage migration inhibitory factor (MIF) deficiency exacerbates aging-induced cardiac remodeling and dysfunction despite improved inflammation: role of autophagy regulation. Sci. Rep. 6:22488. 10.1038/srep22488, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu H., Chen G., Yokoyama M., Hirata K., Augustus A., Kako Y., et al. (2003). Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111, 419–426. 10.1172/JCI16751, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bokov A., Gelfond J., Soto V., Ikeno Y., Hubbard G., et al. (2014). Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 69, 119–130. 10.1093/gerona/glt056, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Shen W., Yao K., Wang H., Liu B., Li T., et al. (2019). Fine-tuning of PGC1alpha expression regulates cardiac function and longevity. Circ. Res. 125, 707–719. 10.1161/CIRCRESAHA.119.315529, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]