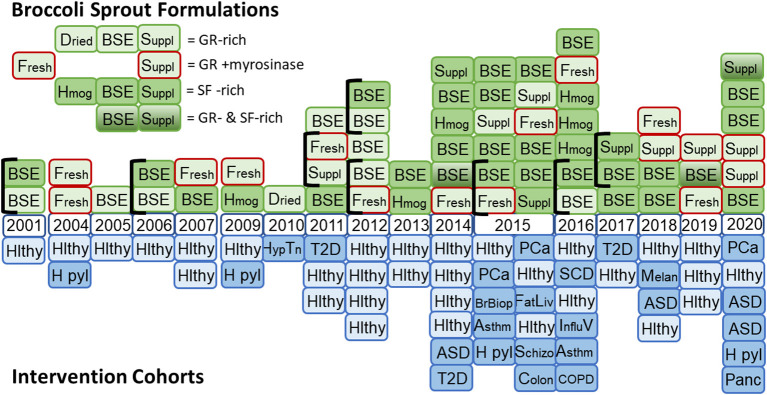
Figure 2.
Timeline for the use of formulations of broccoli sprouts in clinical trials. (Top) Glucoraphanin (GR)-rich preparations included dried broccoli sprouts, hot water broccoli sprout extracts (BSE), and supplements formulated with freeze-dried broccoli sprouts or finely milled broccoli seeds to provide GR. Formulations enclosed with a red border included myrosinase (from either broccoli sprouts or daikon sprouts). Sulforaphane (SF)-rich formulations included fresh homogenates of broccoli sprouts, BSE treated with myrosinase or supplements including freeze-dried broccoli sprouts and finely milled broccoli seeds to provide myrosinase and GR. A few studies included blended formulations that included both GR-rich and SF-rich preparations. (Bottom) Mirrored against the study formulations are the study cohorts used with each formulation. Hlthy, healthy volunteers; H pyl, Helicobacter pylori infected participants; Hyp Tn, hypertensive participants; T2D, type 2 diabetes patients; ASD, autism spectrum disorder patients; PCa, prostate cancer patients; BrBiop, patients undergoing breast biospsy; Asthm, patients with asthma; FatLiv, patients with fatty liver; Schizo, schizophrenia patients; Colon, colon cancer patients; SCD, sickle cell disease patients; Influ V, influenza virus infected participants; COPD, chronic obstructive pulmonary disease; Melan, melanoma patients with multiple atypical nevi; Panc, patients with pancreatic cancer.