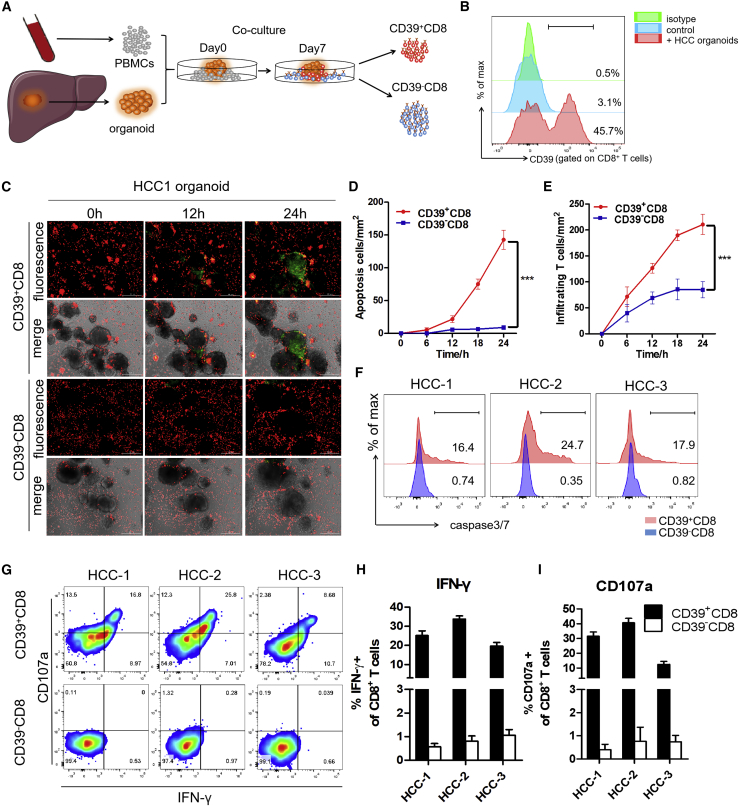
Figure 7.
CD39+ personalized tumor-reactive CD8+ T cells generated from organoid-T cell co-culture system exerted stronger antitumor activity
(A) Experimental design. HCC samples were obtained from a needle biopsy of primary tumors. Organoids were established and co-cultured with autologous peripheral blood lymphocytes (PBLs). After 1 week of co-culture, CD39+/− tumor-reactive CD8+ T cells were expanded and sorted by FACS separately. CD39+/− CD8+ T cells were then co-cultured with autologous tumor organoids for 24 h. The antitumor effect was observed by measuring the apoptotic cells within the tumor organoids. (B) Frequency of CD39 gated on tumor-reactive CD8+ T cells after 1 week of co-culture was evaluated by flow cytometry. No organoids added was used as NC. (C) Tumor-reactive CD8+ T cells (red) were labeled with CellTrace Far Red and apoptotic cells (green) were labeled with caspase3/7 probe. Real-time Biotech imaging system was used to take 1 image per hour for 24 h. Representative images for 1 representative patient were shown. Scale bar, 300 μm. (D and E) Summaries of quantitative statistics of (C). Apoptotic cells (green) and infiltrating tumor-reactive CD8+ T cells (red) inside the area of organoids were calculated by the Spot function of Imaris software based on the size and intensity threshold. The initial number of infiltrating T cells at 0 h was defined as 0. ∗∗∗p < 0.0005 (1-way ANOVA). All data are means ± SEMs. Error bars represent SEMs of 3 view fields from each patient. (F) Representative flow cytometry analysis of organoids apoptosis by caspase3/7 probe (FITC). (G–I) Representative flow cytometry analysis and relative quantification of IFN-γ production and CD107a expression of CD39+/− CD8+ T cells. Error bars represent SEMs of 3 biological replicates.