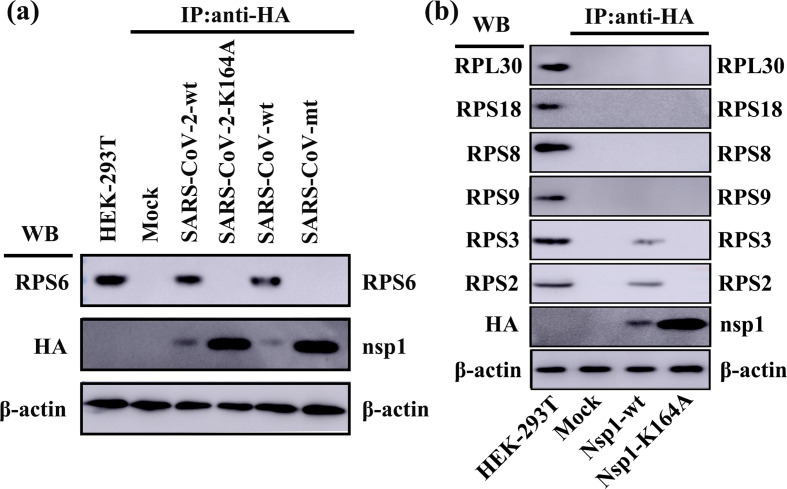
Fig. 5.
SARS-CoV-2 nsp1 K164A abolishes the interaction between nsp1 and the 40S ribosomal subunit in HEK-293T cells. (a) Using PCAGGS-SARS-CoV nsp1 (SARS-CoV-wt) and PCAGGS-SARS-CoV nsp1-K164A/H165A (SARS-CoV-mt) as a control, immunoprecipitated proteins were examined by Western-blot analysis using an anti-RPS6 antibody (40S subunit specific) and anti-HA antibody for PCAGGS (mock), PCAGGS-SARS-CoV-2 nsp1 (SARS-CoV-2-wt) and PCAGGS-SARS-CoV-2 nsp1-K164A (SARS-CoV-2-mt). (b) The immunoprecipitated proteins (RPS2, RPS3, RPS9, RPS8, RPS18 and RPL30) and SARS-CoV-2 nsp1 (Nsp1-wt) or SARS-CoV-2 nsp1-K164A (Nsp1-K164A) were examined by Western-blot analysis using anti-RPS2, anti-RPS3, anti-RPS9, anti-RPS8, anti-RPS18, anti-RPL30 and anti-HA antibody. The expression of β-actin was detected with an anti-β-actin monoclonal Ab to confirm equal protein loading.