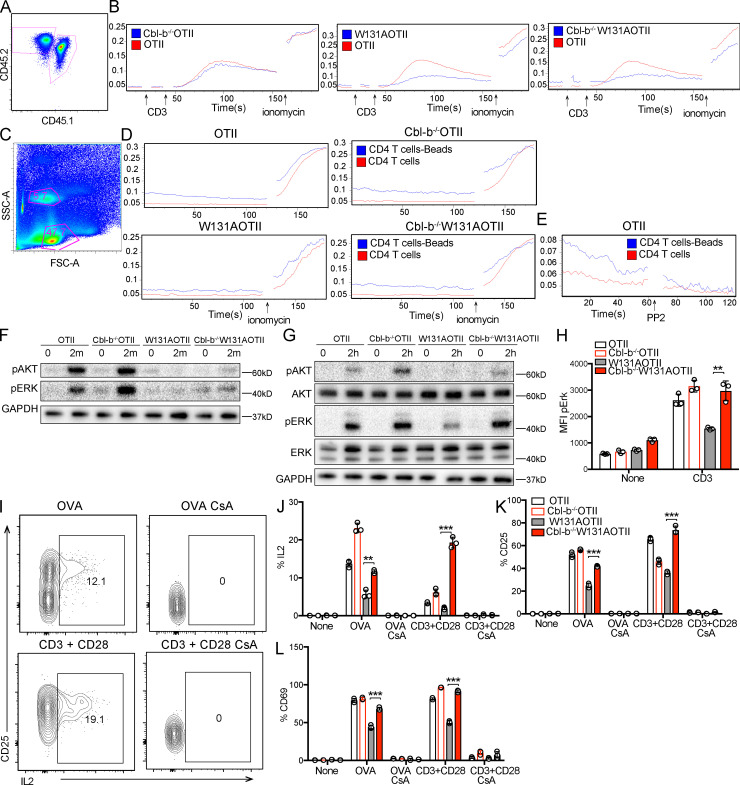
Figure 7.
Loss of Cbl-b in W131AOTII mice rescued calcium responses and TCR signaling. (A) Peripheral T cells were mixed together, identified by congenic markers CD45.1x CD45.2 (OTII) and CD45.2 (Cbl-b−/−W131AOTII or W131AOTII, or Cbl-b−/−W131AOTII), and loaded with Indo-1 to detect intracellular calcium. (B) Cells were stimulated with 10 µg/ml anti-CD3, followed by cross-linking with 20 µg/ml anti–Armenian hamster IgG, and 1 µM ionomycin. (C and D) Peripheral OTII and Cbl-b−/−OTII or W131AOTII or Cbl-b−/−W131AOTII CD4 T cells were culture with anti-CD3/CD28 Dynabeads for 3 h. Cell were loaded with Indo-1 for 30 min to detect intracellular calcium. (C) Flow cytometry shows bead-bound cell binding has higher side scatter (SSC-A) than unbound cells. FSC-A, forward side scatter. (D) Calcium changes in bead-bound and unbound cells. Cells were stimulated with 1 µM ionomycin. (E) Calcium changes in bead-bound and unbound OTII T cells incubated with 10 µM PP2. (F) Immunoblot analysis of AKT and ERK phosphorylation in peripheral naive CD4+ T cells stimulated with anti-CD3 (1 µg/ml) followed by cross-linking with anti–Armenian hamster IgG (20 µg/ml) for 2 min (n = 3–6 mice/group). (G and H) Immunoblot analysis (G) and flow-based assessment (H) of AKT and ERK phosphorylation in peripheral naive CD25−Va2+CD4+ T cells stimulated with plate-bound anti-CD3 (1 µg/ml) for 2 h (n = 3–6 mice/group). (I–L) Peripheral naive CD4+ T cells were cultured with OVA (0.1 µM) with or without cyclosporin A (CsA; 10 µg/ml) or anti-CD3 (0.5 µg/ml) plus anti CD28 (2 µg/ml) with or without CsA (10 µg/ml). (I) Flow cytometry of peripheral CD4+Vα2+ T cells stained for CD25 and IL-2. (J–L) Bar chart indicating mean frequencies ± SD for IL-2+ (J), CD25+ (K), and CD69+ (L) of Vα2+CD4+ T cells (n = 3 mice/group). Data are representative of at least two independent experiments. Two-tailed Student’s t test was performed. **, P < 0.005; ***, P < 0.0005.