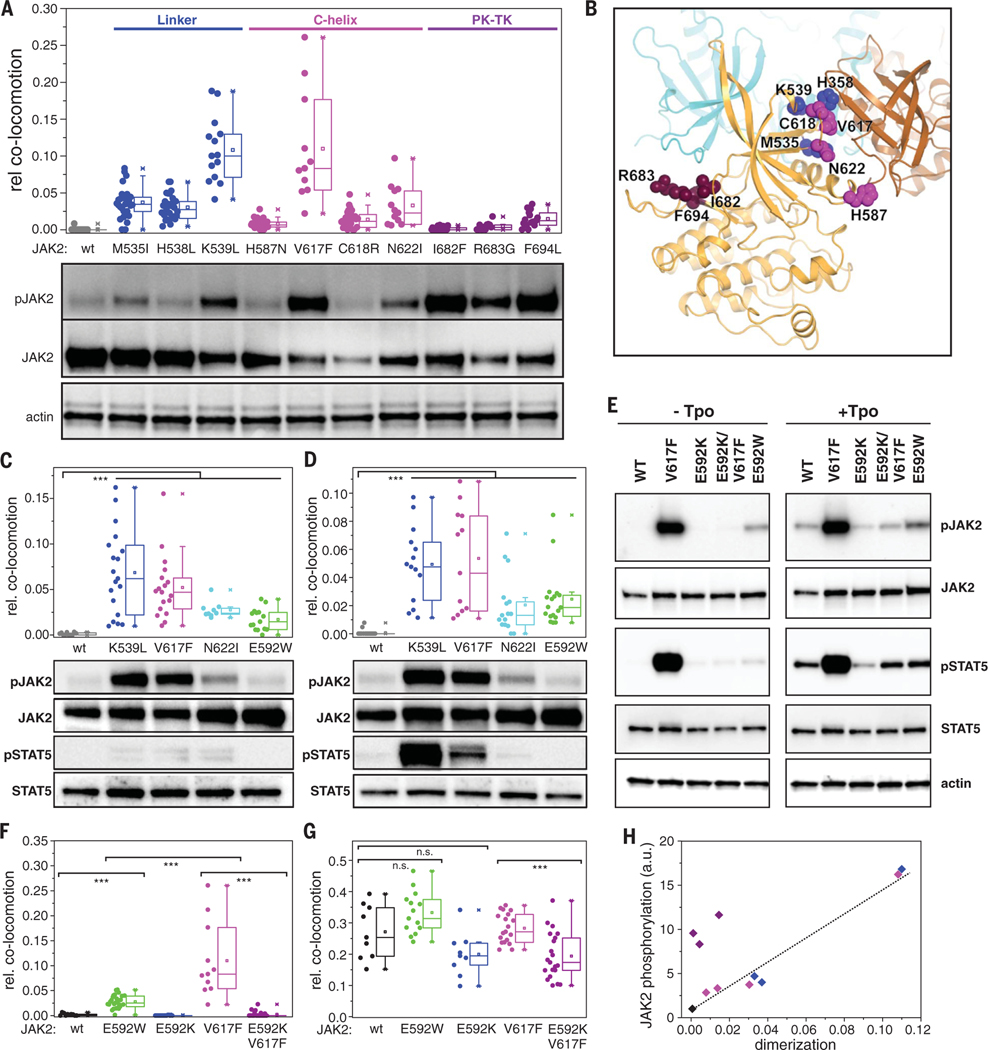
Fig. 5. Dimerization interface of JAK2 PK domains.
(A) Ligand-independent dimerization of TpoR (top) and associated JAK2 phosphorylation (bottom) in the presence of oncogenic mutations within the JAK2 PK domain. Residues are grouped and colored according to their location within the PK structure: FS-PK linker (blue); αC helix (magenta), and PK-TK interface (purple). (B) Putative intermolecular PK-PK interface derived from the MD simulations, with one PK domain colored orange and the other brown. The positions of the residues mutated in (A) are mapped onto the orange PK domain. Superimposed in cyan is the TK domain in its autoinhibitory configuration (intramolecular) relative to the orange PK domain; the TK domain would clash with the second (brown) PK domain. (C and D) Ligand-independent dimerization of EpoR (C) and GHR (D) (top) and associated JAK2 phosphorylation (bottom) for selected constitutively active JAK2 mutants. (E to G) Altering dimerization and activation by perturbation of the putative PK-PK interface via mutagenesis of Glu592. (E) Activity of different JAK2 mutants in HeLa cells stably expressing mXFP-TpoR. Phosphorylation of JAK2 and STAT5 in the absence of ligand (left) and after stimulation with Tpo (right) was probed by Western blot. [(F) and (G)] Dimerization of TpoR associated with different JAK2 mutants in the absence (F) and presence (G) of Tpo. (H) Correlation of receptor dimerization with activation for constitutively active JAK2 mutants [same color coding as in (A)]. Error bars are omitted for clarity. In (A), (C), (D), (F), and (G), each data point represents the analysis from one cell with a minimum of nine cells measured for each condition. ***P ≤ 0.001.