Alcoba-Florez and colleagues [1] recently reported the local transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of the lineage B.1.1.7 in Tenerife, Spain. This should be a cause for concern because based on current observations, among patients with coronavirus disease 2019 (COVID-19), SARS-CoV-2 of the lineage B.1.1.7 persisted longer in the respiratory tract of infected patients and attained higher viral RNA loads compared to those of other variants [2,3]. Therefore, the findings indicate the possibility for more severe illness and higher rate of mortality apart from higher transmissibility, in patients infected with SARS-CoV-2 of the lineage B.1.1.7, compared to other variants [2,3]. There had been attempts to investigate if the mortality rate of patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 is higher compared to those infected with other variants. Therefore, we aimed to perform a meta-analysis to summarize the overall risk of mortality in patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 relative to their counterparts infected with other variants.
We performed a systematic literature search with no language restriction in electronic databases, which included PubMed, Google Scholar, Scopus, and preprint servers (medRxiv, Research Square, SSRN), to identify for published studies, dated up to April 21, 2021. The search strategy was built based on the following keywords and MeSH terms: “COVID-19″, “SARS-CoV-2″, “b.1.1.7″, “202012/01″ and "202012/1". Studies eligible for inclusion were observational studies comparing the risk of mortality between patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 and those infected with other variants. Two investigators (CSK and SSH) independently performed the literature screening to identify eligible studies. The outcome of interest was all-cause mortality.
Two investigators (CSK and SSH) extracted the study characteristics, which included the first author's surname, country of origin, study methods, study population, and mortality estimates. Meta-analyses with the random-effects model were used to estimate the pooled hazard ratio of mortality in patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 relative to their counterparts infected with other variants, at 95% confidence intervals. We examined the heterogeneity between studies using the I2 statistics and the χ2 test, with significant heterogeneity predetermined at 50% and P<0.10, respectively. All analyses were performed using Meta XL, version 5.3 (EpiGear International, Queensland, Australia).
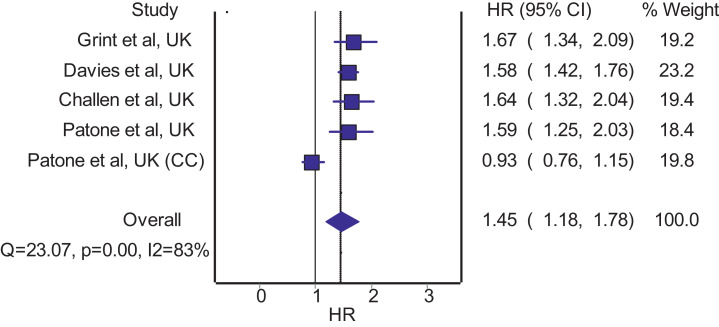
Our systematic literature search retrieved 265 hits, of which 207 were unique (titles retrieved after removing duplications). After screening against eligibility criteria, four observational studies 4, 5, 6, 7 were included, with a total of 2,738,113 patients with COVID-19. All included studies 4, 5, 6, 7 were originated from the United Kingdom. Table 1 summarizes the characteristics of the four included studies 3, 4, 5, 6 which evaluated the risk of mortality between patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 and those infected with other variants. The meta-analysis of four studies with mortality estimates as hazard ratio indicates significantly increased hazard of mortality (Fig. 1 ; pooled hazard ratio=1.45; 95% confidence interval: 1.18 – 1.78) among patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 relative to those infected with other variants. The pooled hazard ratio remained significant even after excluding the critical care cohort of patients from the study by Patone et al. [7] (pooled hazard ratio=1.60; 95% confidence interval: 1.47 – 1.74).
Table 1.
Characteristics of published studies evaluated the risk of mortality between patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 and those infected with other variants.
| Study | Country | Method | Sample | Total number of patients | Outcome | Estimate of effect (95% confidence interval) |
|---|---|---|---|---|---|---|
| Grint et al. [5] | United Kingdom | Cox proportional hazards regression model | Cases who tested positive for SARS-CoV-2 between 16 November 2020 and 11 January 2021 identified from OpenSAFELY electronic health records secure research platform | 184,786 | Hazard ratio for death for VOC-infected individuals to non-VOC infected individuals |
1.67 (1.34–2.09) |
| Davies et al. [6] | United Kingdom | Cox proportional hazards regression model | Cases who tested positive for SARS-CoV-2 between 1 November 2020 and 14 February 2021 identified from Public Health England datasets, adjusted for misclassification of SGTF and missingness of data |
2,245,263 | Hazard ratio for death for VOC-infected individuals to non-VOC infected individuals |
1.58 (1.42–1.76) |
| Challen et al. [7] | United Kingdom | Matched cohort study | Cases older than 30 years who tested positive for SARS-CoV-2 between 1 October 2020 to 29 January 2021 in the community-based (pillar 2) COVID-19 testing centres and with known S gene status, matched with age, date of specimen collection, sex, ethnicity, geographical location, and index of multiple deprivation | 109,812 | Hazard ratio for death for VOC-infected individuals to non-VOC infected individuals |
1.64 (1.32–2.04) |
| Patone et al. [8] | United Kingdom | Flexible parametric survival model (Royston-Parmar) |
Patients in primary care who tested positive for SARS-CoV-2 between 1 November 2020 and 26 January 2021 identified from QResearch data platform | 198,420 | Hazard ratio for death for VOC-infected individuals to non-VOC infected individuals |
1.59 (1.25–2.03) |
| Patients admitted for critical care who tested positive for SARS-CoV-2 between 1 November 2020 and 27 January 2021 identified from QResearch data platform | 3432 | 0.93 (0.76–1.15) |
Fig. 1.
Pooled hazard ratio for mortality in patients with COVID-19 infected with SARS-CoV-2 of the lineage B.1.1.7 compared to those infected with other variants.
The evidence to date pointed towards increased risk of mortality in patients infected with SARS-CoV-2 of the lineage B.1.1.7, compared to other variants, which might be due to the modified viral dynamics as mentioned beforehand [2,3]. In addition, increased binding affinity between the Spike receptor-binding domain and angiotensin-converting enzyme 2 (ACE2) receptor with SARS-CoV-2 of the lineage B.1.1.7 as previously reported could lead to more ACE2 downregulation should an individual acquire this new variant compared to other variants; ACE2 might have a protective effect against lung injury in patients with COVID-19 [8,9]. The patients contracting COVID-19 should be screened for the acquisiton of B.1.1.7 variant; those infected with this variant of concern should be clinically monitored early in the disease course so that an aggressive therapeutic intervention can be offered in time to reduce the risk of mortality [10].
Declaration of Competing Interest
All authors report no conflicts of interest relevant to this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Reference
- 1.Alcoba-Florez J., Lorenzo-Salazar J.M., Gil-Campesino H. Monitoring the rise of the SARS-CoV-2 lineage B.1.1.7 in Tenerife (Spain) since mid-December 2020 [published online ahead of print, 2021 Apr 20] J Infect. 2021 doi: 10.1016/j.jinf.2021.04.005. S0163-4453(21)00168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calistri P., Amato L., Puglia I. Infection sustained by lineage B.1.1.7 of SARS-CoV-2 is characterised by longer persistence and higher viral RNA loads in nasopharyngeal swabs [published online ahead of print, 2021 Mar 5] Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.03.005. S1201-9712(21)00210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Yan L.M., Wan L. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grint D.J., Wing K., Williamson E. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill. 2021;26(11) doi: 10.2807/1560-7917.ES.2021.26.11.2100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies N.G., Jarvis C.I. CMMID COVID-19 Working Group, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7 [published online ahead of print, 2021 Mar 15] Nature. 2021 doi: 10.1038/s41586-021-03426-1. 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patone M., Thomas K., Hatch R., San Tan P., Coupland C., Liao W., et al. Analysis of severe outcomes associated with the SARS-CoV-2 Variant of Concern 202012/01 in England using ICNARC Case Mix Programme and QResearch databases. Preprint. medRxiv. 2021;2021.03.11.21253364.
- 8.Lei, Y., Zhang, J., Schiavon, C.R., et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. bioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 9.Zhang X., Li S., Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad Med J. 2020;96(1137):403–407. doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kow C.S., Hasan S.S. Could it be that the B.1.1.7 lineage is more deadly? Infect Control Hosp Epidemiol. 2021;1 doi: 10.1017/ice.2021.59. [published online ahead of print, 2021 Feb 9] [DOI] [PMC free article] [PubMed] [Google Scholar]