Abstract
We report the case of a patient with a complete metastatic adrenal response on Pembrolizumab for metastatic lung cancer. Treatment with a systemic corticosteroid‐induced a time‐dependent progression at his metastatic site. Surprisingly, after stopping the corticosteroid, we observed a new complete response in long‐term adrenal metastases.
Keywords: case reports, immunotherapy, lung neoplasms, programmed cell death 1 receptor, tumor escape
The administration of systemic corticosteroid therapy for a prolonged period in patients undergoing treatment with immune checkpoint inhibitors for metastatic lung cancer can have a direct impact on the tumor response and may be reversible.
1. INTRODUCTION
Avoiding immune destruction is one of the emerging hallmarks of cancer described by Hanahan and Weinberg in 2011.1 Among the many mechanisms allowing tumor escape from the immune system, membrane receptors involved in the immunomodulation between cancer cells and T lymphocytes have a major role in these mechanisms. These membrane receptors are components of the immune checkpoint pathways that are involved in self‐tolerance and immune response modulation.2
Immune Checkpoint Inhibitors (ICI) are currently used for the management of many cancers. For instance, Pembrolizumab, an anti‐programmed death 1 (PD‐1) antibody, is proposed to patients with metastatic Non‐Small‐Cell Lung Cancer (NSCLC) as first‐line treatment alone or in combination with platinum‐based chemotherapy.3, 4
Systemic corticosteroid therapy is frequently used in thoracic oncology for the management of immunotherapy adverse events and also of secondary brain lesions, epiduritis, respiratory symptoms, or deterioration of the general condition. Some clinical studies showed a putative negative impact on survival for patient treated by ICI with co‐administration of corticosteroids.5, 6
Moreover, several preclinical studies in mice showed that administration of dexamethasone strongly reduces the CD8+ cell/Treg ratio in blood, and such reduction occurred all the time under corticosteroid treatment. Importantly, the CD8+ cell/Treg ratio decreased is concomitant with a negative effect in the anti‐PD‐1 response rate.7
Here, we report the case of a patient with metastatic NSCLC who was treated with Pembrolizumab as first‐line therapy. Due to the presence of side effects, the patient required systemic corticosteroid therapy that had a direct and time‐dependent negative impact on the global response to immunotherapy.
2. CASE REPORT
In December 2017, middle lobe lung adenocarcinoma was detected in a 64‐year‐old man by bronchial endoscopy. The biopsy showed a poorly differentiated adenocarcinoma with solid architecture, 70% of programmed death‐ligand 1 (PD‐L1) positive cells (Tumor Proclone 22C3 Dako pharmDx kit), and a G12A KRAS mutation. Computer tomography (CT) examination revealed a disseminated disease with contralateral pulmonary nodes, mediastinal lymph nodes, and one left adrenal gland metastasis, no brain metastasis was revealed (Figure 1A). The patient had no history of risk factors as professional exposure to carcinogens but reported weaned smoking (40 pack‐years).
FIGURE 1.
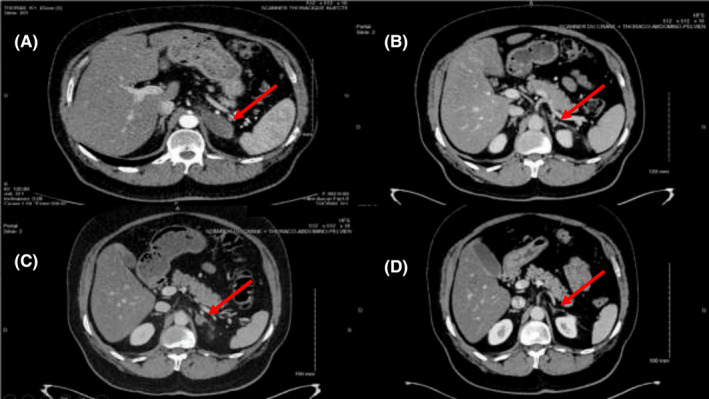
Evaluation of disease progression by CT scan at (A) Baseline scan before treatment with Pembrolizumab, (B) After 6 injections of Pembrolizumab, (C) After 22 injections of Pembrolizumab and the recent corticosteroid therapy, (D) After 24 injections of Pembrolizumab. Red arrows show the left adrenal gland
First‐line treatment with Pembrolizumab 200 mg intravenously (IV) every 3 weeks as monotherapy was started in December 2017. After two injections, the CT scan showed a partial response of all target lesions. After the third injection, 2 months after treatment initiation, the patient presented a maculopapular rash on the back, chest, and legs with widespread pruritus (grade 2 toxicity) (Figure 2). Pembrolizumab was continued, and after six injections, complete response of the adrenal gland metastasis was observed, while the thoracic disease was stable (Figure 1B). Concomitantly, recurrent skin rash episodes with intermittent pruritus were reported by the patient.
FIGURE 2.
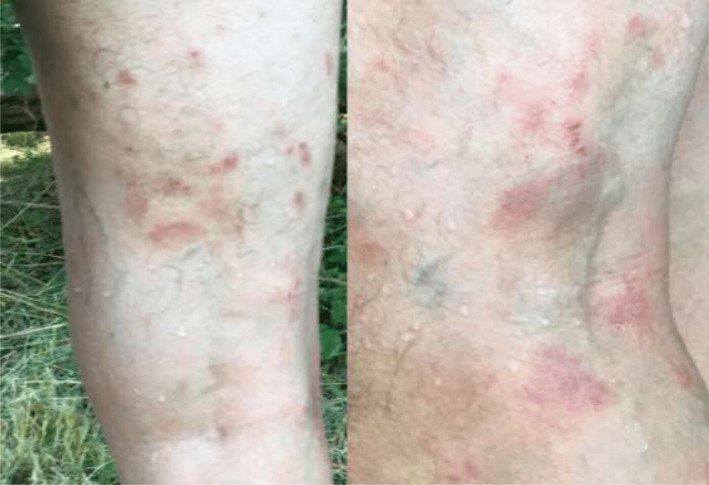
Maculopapular rash of the legs
After 15 injections of Pembrolizumab, evaluation of the residual disease by positron emission tomography showed low uptake in the right hilar region and mediastinal nodes, and persistent complete response of the adrenal metastasis. Pulmonary nodes were not detectable. Therefore, the patient underwent mediastinal and hilar radiotherapy in the first 2 weeks of December 2018. On December 20, the patient started systemic corticosteroid therapy with prednisolone (20 mg/d) because of invalidating pruritus (Figure 3). The patient developed dyspnea and fever and was hospitalized from December 31, 2018 to January 3, 2019. The CT scan showed alveolar interstitial syndrome, suggesting radiation pneumonitis. Thus, prednisolone dose was increased to 80 mg/d. Finally, the patient quickly improved clinically and radiologically after a probabilistic antibiotic treatment for seven days. The diagnosis of infectious pneumopathy was finally retained rather than radiation pneumonia, although no bacteria could be detected. Pembrolizumab was continued (Figure 3) and under prednisolone, pruritus disappeared, and the cutaneous rash improved.
FIGURE 3.
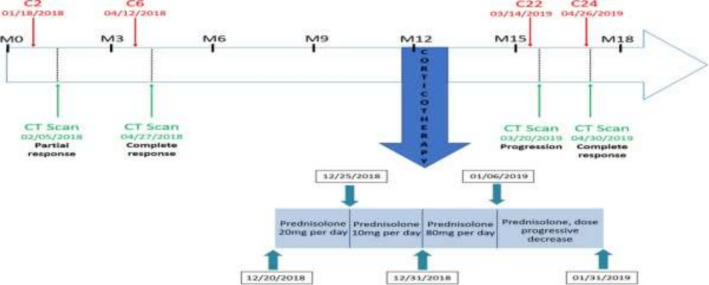
Changes of the adrenal gland metastasis status over time. Cn, number of Pembrolizumab injections; M, month after NSCLC diagnosis. Blue rectangles depict the corticosteroid schedule
In March 2019, after 22 injections of Pembrolizumab and corticosteroid therapy for 6 weeks, the CT scan revealed, for the first time, progression of the left adrenal gland metastasis, while the thoracic disease remained stable (Figure 1C). Two months later, the CT scan showed again complete response of the adrenal gland metastasis, while the skin rush reappeared (Figure 1D). Since then, the complete tumor response has persisted.
3. DISCUSSION
This patient presented a grade 2 skin rash during treatment with Pembrolizumab that can be considered as a predictive factor of his prolonged response (currently still under immunotherapy: Injection number 41 performed 04/28/2020). The apparent factor leading to dissociated response of the adrenal gland metastasis was systemic corticosteroid therapy for 6 weeks at a dose >10 mg/d of prednisolone. This recurrence rapidly regressed, after stopping corticosteroids, as indicated by the CT findings 2 months later.
We have not observed any thoracic tumor progression following corticosteroid therapy. This can be explained by the fact that thoracic radiotherapy was carried out a few weeks before the start of systemic corticotherapy, which allowed local control of the tumor. This patient had an oligo‐metastatic presentation, so, unfortunately, we cannot, therefore, present an occurrence of progression under corticosteroid at another tumor site.
ICI is associated with Immune‐Related Adverse Events (irAEs).8 The correlation between ICI response rate and irAE occurrence is not a recent finding. Clinicians observed early that tumor response was higher in patients with grade III/IV irAEs than without irAEs.9 Several studies showed that in patients with advanced NSCLC treated with Nivolumab, another anti‐PD‐1 antibody, irAE occurrence was correlated with a clear improvement in progression‐free survival (PFS) and overall survival (OS).10, 11, 12 Skin irAEs of any grade occur in 14%‐47% of patients treated by ICI,13 and seem to be independently associated with better PFS and OS when they occur during treatment with Pembrolizumab.14, 15
The management of irAE can be difficult. Many academic institutions offer recommendations for good clinical practice. The European Society for Medical Oncology (ESMO) has proposed recommendations in 2017.16 The cornerstone of irAE treatment is corticosteroids. For the cutaneous, digestive or hepatic irAEs, corticosteroids are introduced in the case of an adverse event of grade ≥3 or persisting grade 2.
In the case of immune‐related pneumonitis toxicities, treatment with corticosteroids is recommended starting from a grade 2 adverse event, in the absence of any argument for an infectious etiology. Treatment by systemic corticosteroid therapy in the case of confirmed irAE is then recommended for a prolonged period that may extend over several months.
While irAE occurrence appears to be strongly correlated with improved OS, the impact of systemic corticosteroid therapy on ICI efficacy remains controversial. In the main trials that tested anti‐PD‐1 antibodies in NSCLC, patients who required treatment with >10 mg of prednisone per day (or steroid equivalent, excluding inhaled or topical steroids) were excluded. Therefore, the interactions between these treatments could not be assessed.3, 17, 18 However, the use of systemic corticosteroids is common in patients treated with anti‐PD‐1 antibodies for NSCLC. For instance, Leighl et al reported that 71/550 patients receiving Pembrolizumab had irAEs, and 42% of them required corticosteroid treatment for their irAE.18
Analysis of the data from retrospective studies highlights two clinical contexts where the effect of systemic corticosteroid therapy appears to be different: (a) at initiation or within the first 30 days of ICI treatment or (b) after the first 30 days for irAEs. The first situation concerns 12%‐20% of patients, depending on the series, and the main indications are respiratory symptoms, fatigue, or brain metastases. In multivariate analyses, this corticosteroid therapy is associated with an increased risk of death, with a hazard ratio = 2.30, 95% CI (1.27‐4.16), P = .006) in the study by Scott and Pennell,19 and a hazard ratio = 1.66, 95% CI (1.28‐2.16), P < .001) in the study by Arbour et al.20 For patients with corticosteroid after 30 days of ICI, two studies on patients with advanced NSCLC treated with Nivolumab found that 15%‐20% of patients presented irAEs that required corticosteroid therapy. Survival rate was comparable between patients with and without corticosteroid for irAEs (73% versus 71% at 6 months and 46% versus 45% at 18 months).21 Similarly, the median OS was not significantly different between patients with and without corticosteroid therapy for irAEs: 16.1 months (95% CI: 7.6‐18.5) and 10.5 months (95% CI: 8.6‐12.2) (P = .50).22
These data suggest that the effect of systemic corticosteroid therapy on survival of patients undergoing ICI treatment is influenced by the timing relative to ICI initiation and the reason for corticosteroid administration. It is obvious that a patient with symptomatic brain metastases at ICI start does not have the same natural history as a patient with a maculopapular rash‐like irAE after several antibody injections. Moreover, although OS does not seem to be affected by corticosteroid therapy for irAEs, irAE occurrence could be a confounding factor that hides the deleterious effect of corticosteroid therapy. As all studies on the influence of systemic corticosteroid therapy on ICI response are retrospective, this question could be better addressed by stratifying patients according to their steroid intake in clinical trials that evaluate ICI efficacy.
This case shows that the introduction of systemic corticosteroid therapy (>10 mg/d) for an irAE that appeared after the first 30 days of ICI can influence the tumor response. This effect appears to be dependent on the duration of corticosteroid administration and it was reversible. Indeed, some groups showed that the dose and the duration of corticosteroid therapy are key factors, and that corticosteroid therapy >10 mg/d for more than 2 weeks could have a negative impact on patient survival, regardless of the starting time.23
Overall, this case suggests that the occurrence of skin irAEs in patients treated with Pembrolizumab for advanced NSCLC is a factor of good prognosis and that the impact of systemic corticosteroid therapy on ICI efficacy although remains to be demonstrated it should be carefully evaluated.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Thomas QD.: took part in the design, the writing and the collection of all the data of this case report, had full access to all of the data in the case report and took responsibility for the integrity of the data. Sinoquet L.: participated in the data collection and writing of the article. Maraver A. and Quantin X.: participated in the design and proofreading of this article.
ETHICS STATEMENT
Published with written consent of the patient.
ACKNOWLEDGMENTS
We would like to thank the patient for participating in this box report. We would like to thank the Cancer Institute of Montpellier for the logistical support in the achievement of this project. We would like to thank Chambrade M. for her support in the development of this report.
Thomas QD, Sinoquet L, Maraver A, Quantin X. Dissociated response related to corticosteroids in lung cancer treated by immunotherapy: A case report. Clin Case Rep. 2021;9:e03973. 10.1002/ccr3.3973
Funding information
None
DATA AVAILABILITY STATEMENT
All data are available on the basis of the Institute of Cancerology of Montpellier and Thomas QD. Provides full access to all of the data.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 5.Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86‐92. [DOI] [PubMed] [Google Scholar]
- 6.Chasset F, Pages C, Biard L, et al. Single‐center study under a French Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic melanoma: negative impact of baseline corticosteroids. Eur J Dermatol EJD. 2015;25(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell R, Luksik AS, Garzon‐Muvdi T, et al. Contrasting impact of corticosteroids on anti‐PD‐1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473‐486. [DOI] [PubMed] [Google Scholar]
- 9.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti‐cytotoxic T‐lymphocyte antigen‐4. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(25):6043‐6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haratani K, Hayashi H, Chiba Y, et al. Association of immune‐related adverse events with nivolumab efficacy in non–small‐cell lung cancer. JAMA Oncol. 2018;4(3). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6583041/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toi Y, Sugawara S, Kawashima Y, et al. Association of immune‐related adverse events with clinical benefit in patients with advanced non‐small‐ cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune‐related adverse events on survival in patients with advanced non‐small cell lung cancer treated with nivolumab: long‐term outcomes from a multi‐institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479‐485. [DOI] [PubMed] [Google Scholar]
- 13.Sibaud V, Boulinguez S, Pagès C, et al. Dermatologic toxicities of immune checkpoint inhibitors. Ann Dermatol Venereol. 2018;145(5):313‐330. [DOI] [PubMed] [Google Scholar]
- 14.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aso M, Toi Y, Sugisaka J, et al. Association between skin reaction and clinical benefit in patients treated with anti‐programmed cell death 1 monotherapy for advanced non‐small cell lung cancer. Oncologist. 2020;25(3):e536‐e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(suppl_4):iv119‐iv142. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2018;13(11):1771‐1775. [DOI] [PubMed] [Google Scholar]
- 20.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(28):2872‐2878. [DOI] [PubMed] [Google Scholar]
- 21.Higashiyama RI, Horinouchi H, Sekine K, et al. Efficacy of nivolumab in patients treated by corticosteroid due to immune‐related adverse events. J Clin Oncol. 2018;36(5_suppl):163. [Google Scholar]
- 22.Leighl N, Gandhi L, Hellmann MD, et al. Pembrolizumab for NSCLC: Immune‐ mediated adverse events and corticosteroid use [abstract]. In: 16th World conference on lung cancer, Denver, CO, 6–9 September 2015, Abstract nr 31.02. New York, NY: Elsevier Science; 2015. [Google Scholar]
- 23.Pan EY, Merl MY, Lin K. The impact of corticosteroid use during anti‐PD1 treatment. J Oncol Pharm Pract. 2019;26(4):814‐822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on the basis of the Institute of Cancerology of Montpellier and Thomas QD. Provides full access to all of the data.


