Abstract
A 27-year-old woman had foggy vision and photophobia since 10 months after implantation of implantable collamer lens (ICL STAAR Surgical AG, Nidau, Switzerland) with evidence of corneal decompensation and no cataract formation. Descemet membrane endothelial keratoplasty in phakic eyes is challenging, considering presence of posterior chamber phakic intraocular lens (IOL), decreasing the space available in anterior chamber to manoeuvre the graft. Need of inferior peripheral iridotomy in presence of central hole technology in ICL depends on the dynamics of full chamber air bubble. At 8 months, vision had improved to 20/20 and normal IOP with well-attached graft, stable phakic IOL and clear lens.
Keywords: anterior chamber, pupil
Background
Endothelial keratoplasty in phakic eyes is challenging. Descemet stripping automated endothelial keratoplasty (DSAEK) in phakic eyes can be associated with shallowing of anterior chamber or pupillary block and risk of cataract formation. On the other hand, descemet membrane endothelial keratoplasty (DMEK) can be managed with good visual outcomes in phakic patients. But in presence of a phakic intraocular lens (IOL) with a clear crystalline lens behind, managing corneal decompensation is surgically challenging. We report a similar case wherein DMEK was performed in presence of a phakic IOL.
Case presentation
A 27-year old woman presented with foggy vision and intolerance to bright light since 10 months with her best-corrected visual acuity (BCVA) in the right eye 20/30 p and 20/20 in the left eye. She was phakic with pre-ICL surgery refraction of −8D sphere and −1D cylinder at 170 in both the eyes with vision improving to 20/25. She underwent implantable collamer lens (ICL, STAAR Surgical AG, Nidau, Switzerland) insertion 10 months ago in the right eye and reported blurry vision from first day postoperative which did not improve. Foggy vision, photophobia, watering and pain in the right eye worsened over a period of 7 months during which she took a break from work and was under conservative management with topical prednisolone 1% in tapering dose, moxifloxacin 0.5% and hypertonic saline NaCL 5% drops. The intraocular pressure was not documented in the previous reports. She was unable to use glasses due to high anisometropia and also did not prefer using contact lenses. Later she referred for the left eye to another surgeon who advised ICL implantation. The left eye did well after the surgery but her vision and symptoms in the right eye did not improve for which she was referred to our cornea clinical services after 3 months. The patient gave history of good vision with glasses, no history of any other ocular surgery or trauma prior to the ICL implantation in both the eyes. There was no family history suggestive of corneal diseases such as dystrophies. On examination, inferior corneal oedema with mircosystic changes in the epithelium, peripheral iris atrophy, normal peripheral anterior chamber depth with no iridoendothelial contact and pigment dispersion on the anterior surface of ICL was noted (figure 1A–C). Fundus examination revealed normal optic discs.
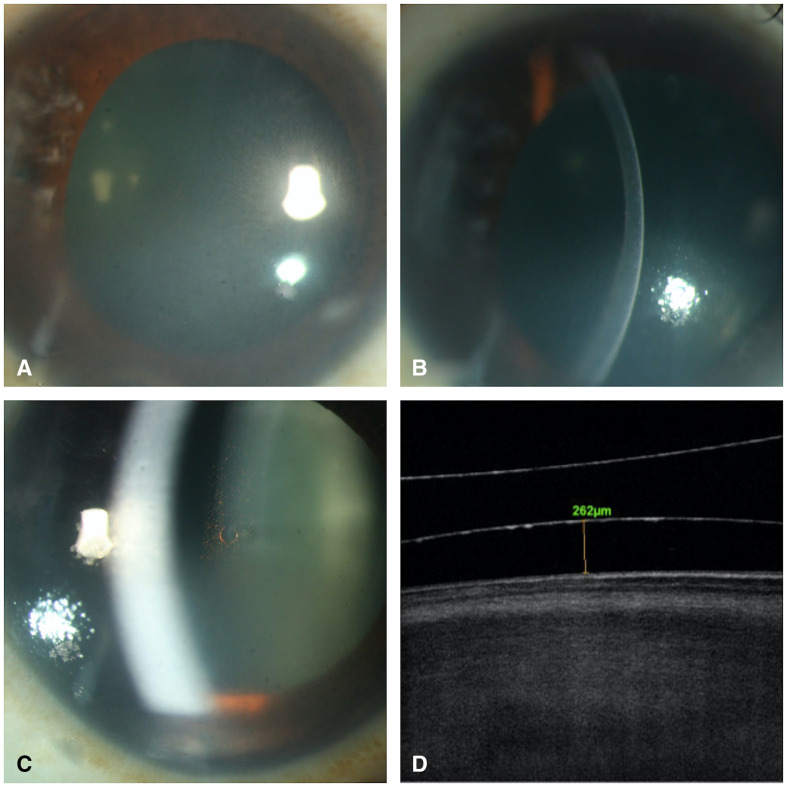
Figure 1.
(A) Diffuse slit lamp image of the right eye showing inferior corneal oedema. (B) Slit lamp image of the right eye showing inferior corneal oedema. (C) ICL in situ. (D) Anterior segment optical coherence tomography of right eye showing vault of ICL in situ. ICL, implantable collamer lens.
Investigations
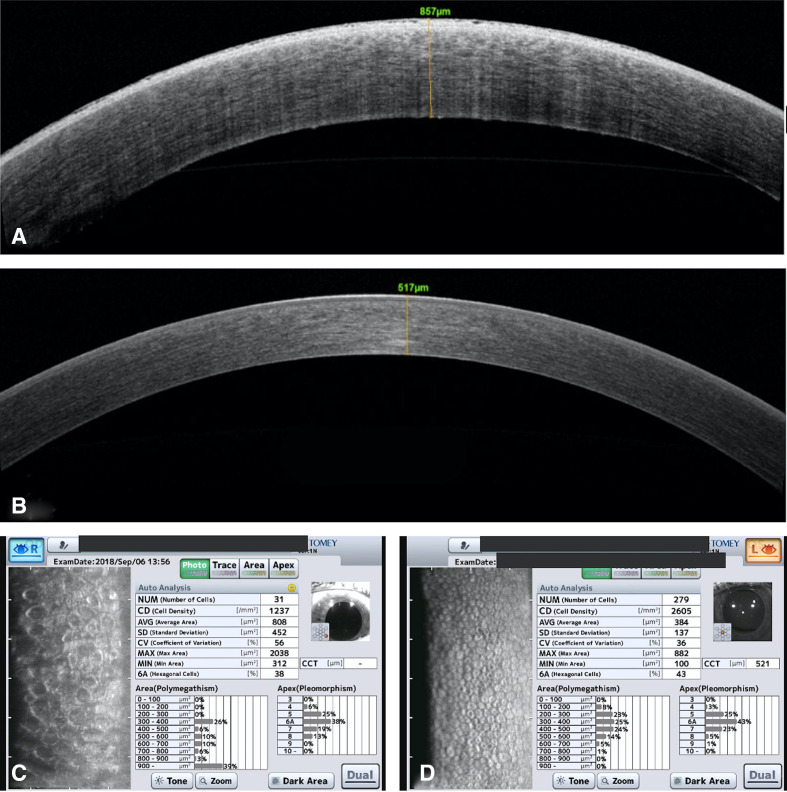
Specular microscopy and anterior segment optical coherence tomography revealed a decreased endothelial cell density (ECD) 1237 cells/mm2 with significant polymegathism, abnormal morphology and increased central corneal thickness (857 μm) in the right eye compared with normal findings in the left eye (ECD 2605 cells/mm2 and central pachymetry 517 μm). There were no guttae in the left eye (figure 2). Intraocular pressure was normal in both the eyes 14 mm Hg in the right eye and 12 mm Hg in the left eye. Her biometry (IOLMaster 700) was suggestive of axial myopia with axial length 26.40 mm in the right eye and 26.85 mm in the left eye with an anterior chamber depth measured on biometry 3.04 mm in the right eye and 2.73 mm in the left eye with ICL in situ.
Figure 2.
(A) AS-OCT of the right eye preoperatively shows corneal oedema and increase in pachymetry. (B) AS-OCT of the left eye shows normal findings. (C) Specular microscopy in the right eye shows corneal decompensation with decreased endothelial cells.(D) Specular microscopy in the left eye shows normal morphology and count of endothelial cells. AS-OCT, anterior segment optical coherence tomography.
Differential diagnosis
Normal morphology of the endothelial cells and absence of corneal signs in the other eye ruled out the possibility of Fuchs endothelial dystrophy. There was neither any history of such an event nor any residual sign in cornea or anterior segment suggestive of a past episode of viral endothelitis. A unilateral presentation and absence of history of exposure to any chemical agents, drugs topically or systemically apart from the routine postoperative medications ruled out toxic endothelitis. Unilateral corneal decompensation along with presence of peripheral iris atrophy and prolonged surgical time can indicate towards multiple intraoperative manipulations. Possibility of wrong orientation of ICL on insertion and attempting to correct the same, inadvertently damaging the endothelium cannot be ruled out. Similarly, pigmentation on anterior surface of ICL may indicate towards severe postoperative inflammatory response such as uveitis or Toxic anterior segment syndrome (TASS), pupillary block leading to increased intraocular pressure or plateau iris syndrome. However, the patient did not give any history of an acute episode of pain or diminution of vision post-ICL implantation and had normal Intraocular pressure(IOP) at presentation with fairly deep peripheral anterior chamber. Although adequate, the vault in the right eye (262 μm) was lesser than the left eye (490 μm). Considering the long-standing decompensation with symptoms and anticipating shallow space during surgery, DMEK was planned over DSAEK.
Treatment
The peculiar challenges of performing a DMEK in this case were to manage a mid-peripheral shallow anterior chamber due to the presence of ICL, to minimise the corneal surface manipulations, to avoid lens touch and air bubble management. It was difficult to maintain an air bubble to perform descemetorhexis which also tended to slip behind the iris and tent it up. As a result, Descemet membrane removal was completed using sodium hyaluronate, which was aspirated thoroughly later. After performing an inferior iridectomy, an 8 mm diameter graft stripped from a 50-year-old donor was inserted into the anterior chamber using a Melles glass tube. The donor graft from a phakic eye had ECD of 2433 cell/mm2. The graft formed a double scroll in correct orientation and corneal tapping with a single canula, unfolded it partially. Due to limited space, the other end was unfolded with difficulty but with gentle and prolonged taps. A full chamber air bubble was injected gently behind the graft after centration and a bandage contact lens (Accuvue Oasys) was placed. The total handling time of the graft in the anterior chamber was 6 min. After 2 hours in supine position, she was discharged when the inferior meniscus of the bubble was above the inferior peripheral iridectom (PI). She was started on topical corticosteroids (prednisolone acetate 1% suspension), antibiotics (moxifloxacin 0.5%) two hourly and cycloplegics (homatropine) three times a day for a week.
Outcome and follow-up
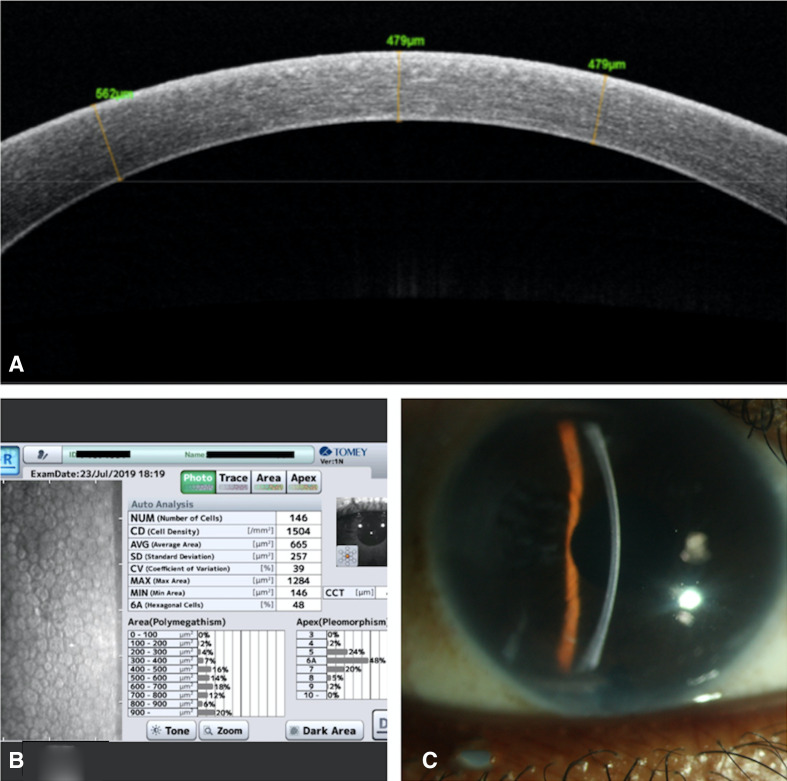
Postoperatively, the graft was attached, well centred with a normal IOP on digital evaluation. At 1 month, BCVA had improved to 20/20 p and the ECD was 1561 cells/mm2 with a reduction of 35.8% cells and IOP of 12 mm Hg, while at 8 months, she had a BCVA of 20/20 and ECD 1504 cells/mm2 with a reduction of 38.18% cells and IOP of 14 mm Hg. The cornea was clear and the ICL was in a normal position with adequate vault and a clear crystalline lens (figure 3).
Figure 3.
(A) Anterior segment optical coherence tomography of the right eye at 8 month postoperative period shows attached Descemets membrane. (B) Right eye endothelial cell count 8 months postoperative. (C) Right eye 8 month postoperative showing Descemets membrane graft is well attached with clear cornea.
Discussion
Corneal decompensation after ICL is a rare complication and has been reported, wherein the endothelial cell loss was delayed and did vary from 9.9% at 2 years to 3.7% at 4 years postoperatively.1
In a case report by Espinossa-Mattar et al, corneal decompensation after ICL implantation occurred due to anterior dislocation of ICL following a blunt trauma and required a DSAEK procedure after 6 months.2 The cause of corneal decompensation in our patient is not evident considering normal findings in other eye, but was probably due to intraoperative manipulation at the time of ICL implantation.
DMEK in a phakic eye is challenging. Siggel et al performed DMEK in seven phakic eyes and had comparable visual outcomes to the published literature; however, the cell loss was higher at 47.6% compared with other studies 32%–36% at 6 months.3 The ECD at 8 months in our case was comparable to the published literature. But presence of a posterior chamber phakic IOL, further decreases the space available in the anterior chamber to manoeuvre the graft during surgery. The configuration of the anterior chamber has a typical mid-peripheral shallowing. There is a risk of inadequate graft unfolding, extra manipulations and graft detachment postoperatively. Positive pressure by the air bubble or excessive manipulations can lead to postoperative cataract formation by pushing the ICL in contact with the crystalline lens. Parker et al published a series of DMEK performed in 52 phakic eyes, and documented good postoperative ECD and visual outcomes compared with pseudophakic eyes.4 They did hypothesise this improvement to the intact optical system in phakic eyes which was also seen in our case. However, they noticed a peculiar complication of misdirection of air bubble below the iris (6/52 eyes) leading to angle closure and secondary cataract (1/52 eyes). In our case, a similar tendency for the bubble to slip behind the iris was noted. Additionally, the requirement of an inferior PI in the presence of an ICL with a central hole is also debatable. Presence of the central hole may help in cases of a pupillary block due to a dislodged air bubble, but since we opted to leave a complete air bubble, the central opening was likely to be blocked by the air bubble, necessitating an inferior PI. Even in presence of a full chamber air bubble, the design of the phakic IOL could maintain its vault and protect the crystalline lens. Using pilocarpine 2% eyedrops 30 min preoperatively or intraoperatively prior to DMEK graft insertion can help constrict the pupil in phakic patients. Although we did not use this technique, it is indeed a good option to avoid DMEK–ICL contact.5 DMEK was the procedure of choice instead of a DSAEK in this case considering a shallow anterior chamber but handling the DMEK scroll in a shallow anterior chamber does require advanced skills and expertise. Another reason for choosing DMEK over DSAEK is the fact that the latter will make the anterior chamber (AC) shallower and in case an ICL removal is needed in the future there will be a risk of iatrogenic graft detachment. ICL has been a very effective and safe procedure to treat patients with higher myopia if good manipulation and safe intraoperative pharmacological products are insured. But in rare complications such as in this case with corneal decompensation in presence of a clear crystalline lens and ICL in situ is a challenging scenario and can be managed with proper preoperative planning, investigations and a DMEK procedure.
Learning points.
In corneal decompensation with a phakic intraocular lens (IOL) in situ, if the crystalline lens is clear, both may be left in situ, as phakic IOLs are considerably stable and need not be removed when a descemet membrane endothelial keratoplasty (DMEK) is planned.
Preoperative planning and selecting an older donor tissue which would help in easy unfolding of Descemets scroll are important in such challenging scenarios.
In presence of central hole technology in the phakic IOL, need of peripheral iridectom during DMEK can be optional and will depend on other clinical findings in the patient.
Acknowledgments
The authors are grateful to Hyderabad Eye Research Foundation.
Footnotes
Twitter: @pravinkrishna
Contributors: Following authors have contributed in the various aspects to complete this work of clinical research. VPJ: design, literature search, data acquisition, data analysis, manuscript preparation and manuscript editing. PKV: concept, definition of intellectual content, clinical study, manuscript review and guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Fernandes P, González-Méijome JM, Madrid-Costa D, et al. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg 2011;27:765–76. 10.3928/1081597X-20110617-01 [DOI] [PubMed] [Google Scholar]
- 2. Espinosa-Mattar Z, Gomez-Bastar A, Graue-Hernández EO, et al. DSAEK for implantable collamer lens dislocation and corneal decompensation 6 years after implantation. Ophthalmic Surg Lasers Imaging 2012;24:e68–72. 10.3928/15428877-20120712-04 [DOI] [PubMed] [Google Scholar]
- 3. Siggel R, Heindl LM, Cursiefen C. Descemet membrane endothelial keratoplasty (DMEK) in phakic eyes with shallow anterior chamber. Graefes Arch Clin Exp Ophthalmol 2015;253:817–9. 10.1007/s00417-014-2850-9 [DOI] [PubMed] [Google Scholar]
- 4. Parker J, Dirisamer M, Naveiras M, et al. Outcomes of Descemet membrane endothelial keratoplasty in phakic eyes. J Cataract Refract Surg 2012;38:871–7. 10.1016/j.jcrs.2011.11.038 [DOI] [PubMed] [Google Scholar]
- 5. Basak SK, Basak S, Gajendragadkar N, et al. Overall clinical outcomes of Descemet membrane endothelial keratoplasty in 600 consecutive eyes: a large retrospective case series. Indian J Ophthalmol 2020;68:1044. 10.4103/ijo.IJO_1563_19 [DOI] [PMC free article] [PubMed] [Google Scholar]