Fig. (3).
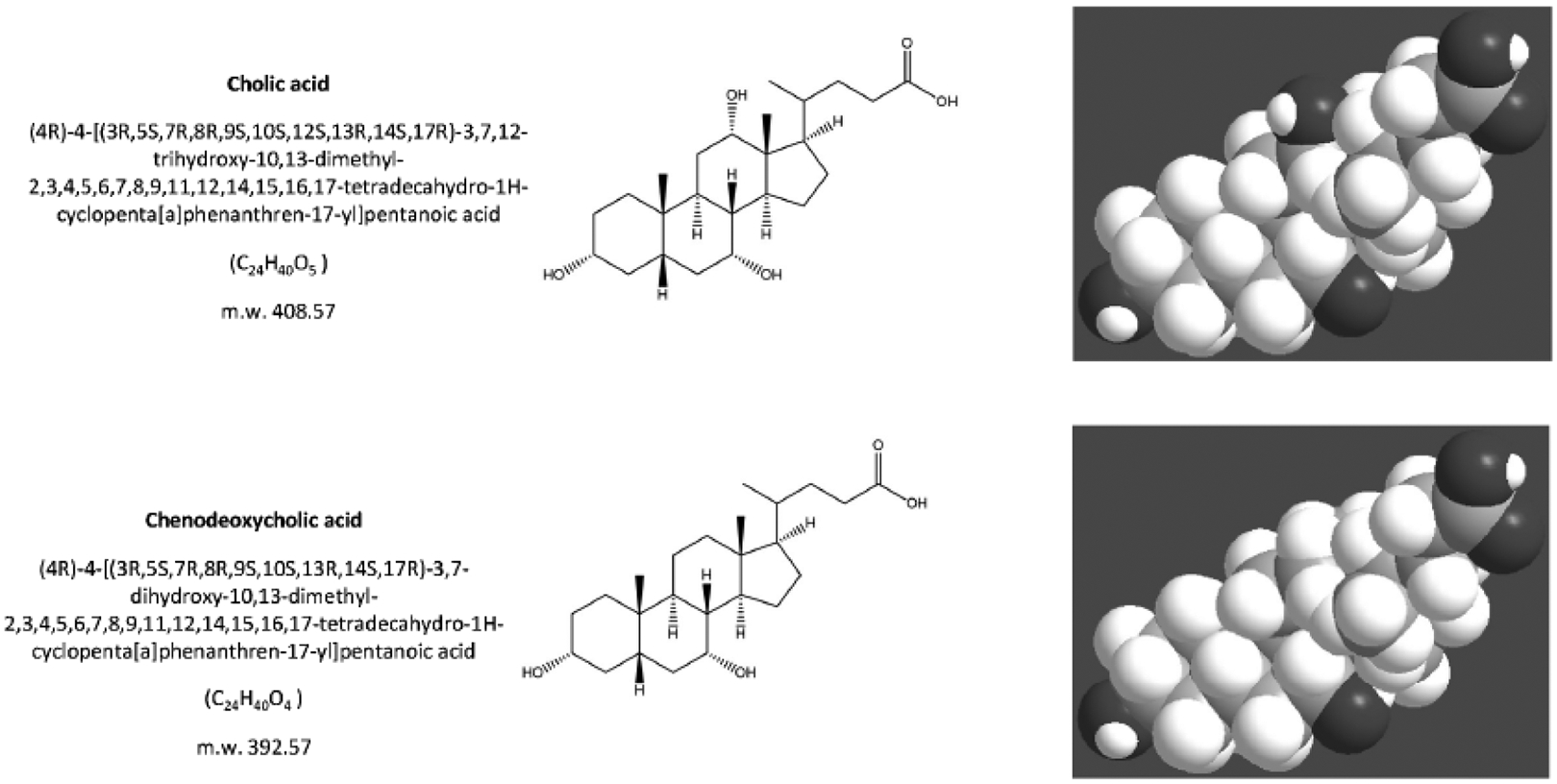
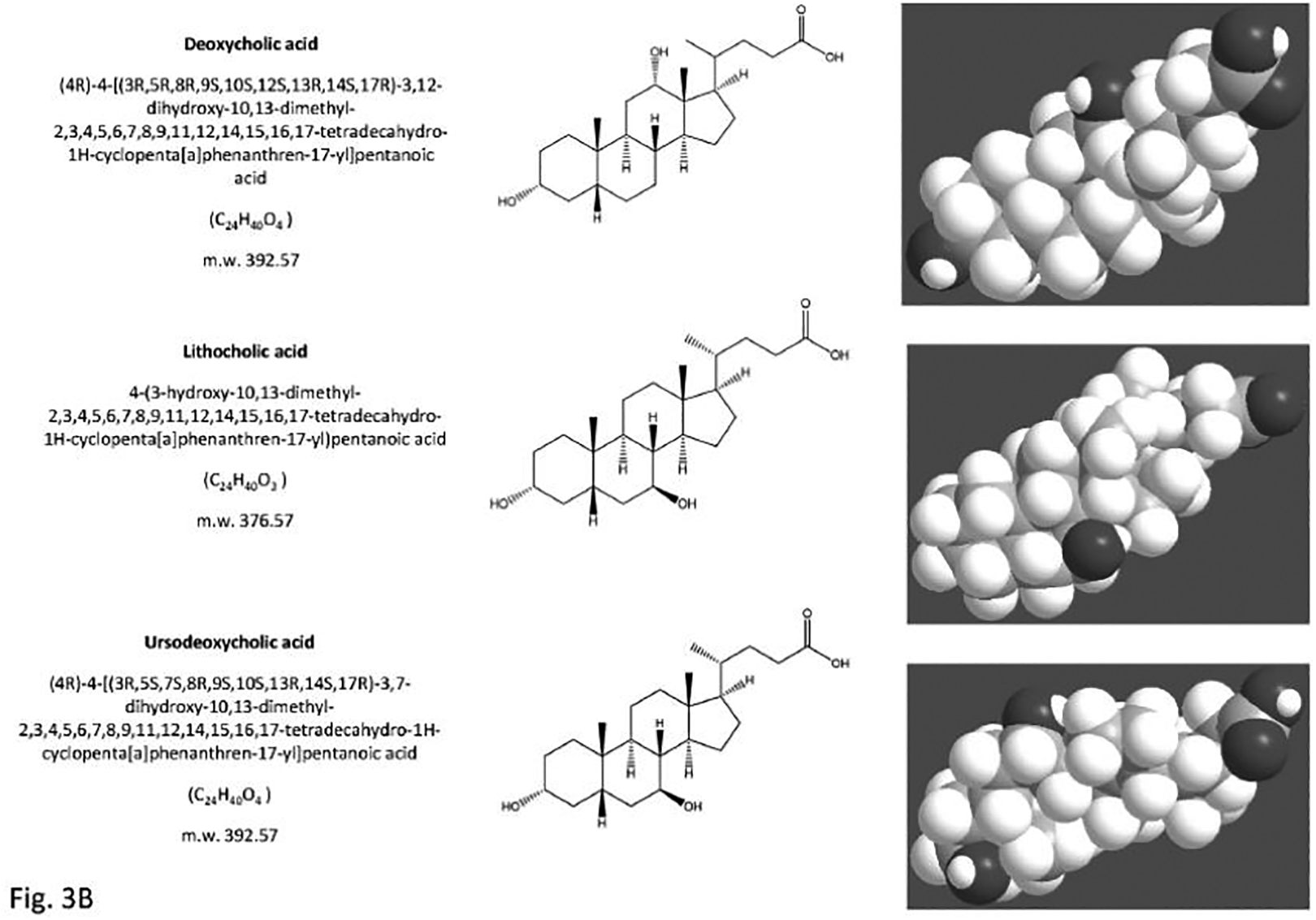
The structure of the primary bile acids, secondary bile acids and “tertiary” bile acid ursodeoxycholic acid. Bile acids are synthesized from cholesterol in the liver as soluble amphiphiles. The biliary bile acid pool in humans is mainly made of the primary bile acids, i.e. the 3,7,12-trihydroxy cholic acid and the 3,7-dihydroxy chenodeoxyholic acid. After being secreted in bile and entering the recirculation in the intestine, the primary bile acids are biotransformed by colonic bacteria into secondary bile acids, i.e. the 3,12-dihydroxy deoxycholic acid (from cholic acid) and the3- monohydroxy lithocholic acid (from chenodeoxycholic acid). “Tertiary” bile acids are the result of modification of secondary bile acids by intestinal flora or hepatocytes. These are the sulfate ester of lithocholic acid and the 3,7-dihydroxy ursodeoxycholic acid (UDCA), and the 7β-epimer of chenodeoxycholic acid. Bile acids are highly soluble, detergent-like amphiphilic molecules; the hydrophilic (polar) areas of bile acids are the hydroxyl groups and conjugation side chain of either glycine or taurine and their hydrophobic (nonpolar) area is the ringed steroid nucleus [64, 192].
