Abstract
Background.
The mismatch negativity (MMN) brainwave indexes novelty detection. MMN to infrequent pitch (pMMN) and duration (dMMN) deviants is reduced in long-term schizophrenia. Although not reduced at first psychosis, pMMN is inversely associated with left hemisphere Heschl’s gyrus (HG) gray matter volume within 1 year of first hospitalization for schizophrenia-spectrum psychosis, consistent with pathology of left primary auditory cortex early in disease course. We examined whether the relationship was present earlier, at first psychiatric contact for psychosis, and whether the same structural-functional association was apparent for dMMN.
Method.
Twenty-seven first-episode schizophrenia-spectrum (FESz) and 27 matched healthy comparison (HC) individuals were compared. EEG-derived pMMN and dMMN were measured by subtracting the standard tone waveform (80%) from the pitch- and duration-deviant waveforms (10% each). HG volumes were calculated from T1-weighted structural magnetic resonance imaging using Freesurfer.
Results.
In FESz, pMMN amplitudes at Fz were inversely associated with left HG (but not right) gray matter volumes, and dMMN amplitudes were associated significantly with left HG volumes and at trend-level with right HG. There were no structural-functional associations in HC.
Conclusions.
pMMN and dMMN index gray matter reduction in left hemisphere auditory cortex early in psychosis, with dMMN also marginally indexing right HG volumes. This suggest conjoint functional and structural pathology that affects the automatic detection of novelty with varying degrees of penetrance prior to psychosis. These brainwaves are sensitive biomarkers of pathology early in the psychotic disease course, and may serve as biomarkers of disease progression and as therapeutic outcome measures.
Keywords: first episode, schizophrenia spectrum, psychosis, mismatch negativity (MMN), Heschl’s gyrus, neurophysiology
Introduction
Functional abnormalities within Heschl’s gyrus (HG), containing primary and parts of secondary auditory cortex, are linked to perceptual abnormalities in schizophrenia such as auditory hallucinations.1–3 HG pathology is also linked to earlier sensory abnormalities such as impaired auditory event-related oscillations4 and auditory event-related potential (ERP) reductions in N1005 and its magnetic counterpart M100,6 and in mismatch negativity (MMN).7 MMN, the ERP difference between a repetitive standard tone and an infrequent deviant tone, is thought to be an automatically generated sensory cortex response to stimulus deviance from a repetitive pattern, or novelty. Detection of novelty is critical for survival via the automatic orienting response, yet also plays a role in navigating the everyday social environment. The process of auditory novelty detection as indexed by the MMN is robustly reduced in schizophrenia.8,9 Meta-analysis, however, suggests the MMN impairment is substantially less at the first episode for schizophrenia.10 More specifically, Haigh et al’s meta-analysis11 showed no evidence for a pitch-deviant MMN (pMMN) reduction in first-episode schizophrenia (FESz), and an effect size for duration-deviant MMN roughly half as large as in long-term illness. The limited longitudinal studies suggest the MMN deficit appears to increase such that by 2 years or so after the emergence of psychosis the MMN is impaired nearly as greatly as in long-term illness7,12 (see Salisbury et al13 for complete discussion of FESz MMN studies).
The major generator of MMN is near primary auditory cortex within Heschl’s gyrus (HG) on the superior temporal plane.14–17 Several longitudinal studies of FESz documented progressive gray matter auditory cortex gray matter loss, more marked in the left hemisphere.18,19 Salisbury and colleagues7 showed that pMMN correlated with left HG gray matter volume at first hospitalization for schizophrenia-spectrum psychosis, with smaller pMMN associated with less left HG gray matter. They argued that pMMN thus indexed the degree of pre-psychosis left auditory cortex gray matter loss. Furthermore, with longitudinal assessment following the onset of schizophrenia-spectrum psychosis, pMMN became reduced in tandem with reductions in gray matter volumes in left HG but not right. Based on these structural-functional associations, the authors proposed that pMMN was a biomarker of disease progression near psychosis onset.
The aim of this study was to replicate the finding of correlation between pMMN and left HG gray matter in a larger independent sample even earlier in disease course, at first clinical contact for a putative schizophrenia-spectrum psychotic disorder, and extend the research by determining the relationship between dMMN and HG gray matter volumes. As mentioned above, meta-analyses indicate that dMMN is more affected than pMMN in FESz, and within long-term schizophrenia, dMMN appears to be most sensitive to the disorder.20 Thus, in concert with the previously demonstrated specific left HG association with pMMN,7 we predict that both pMMN and dMMN will be associated with left HG volumes in FESz but not right.
Methods
Participants
All FESz participants were recruited from Western Psychiatric Hospital (WPH) inpatient and outpatient services. Twenty-seven FESz were compared with 27 healthy controls (HC). FESz consensus diagnoses included schizophrenia (9 paranoid, 3 undifferentiated, and 2 residual) schizoaffective disorder (5 depressed subtype), schizophreniform disorder (2), and psychosis not otherwise specified (6). FESz participated within 2 months of their first clinical contact for a first episode of psychosis and had less than 2 months of lifetime antipsychotic medication exposure. Ten FESz (37%) were unmedicated. HC were recruited from the local community through advertisements. All participants were between the ages of 14 and 40 years. All subjects had normal hearing as assessed by audiometry and at least nine years of schooling, and all reported good physical health. None of the participants had a history of concussion or head injury with sequelae, history of alcohol or drug dependence, detox in the past 5 years, or neurological comorbidity. Groups were matched for age, gender, Wechsler Abbreviated Scale of Intelligence (WASI) IQ, and parental socioeconomic status. Demographic, neuropsychological, and clinical information are presented in Table 1. All participants provided informed consent, and were paid for participation. All procedures were approved by the local institutional review board.
Table 1.
Demographic, Neuropsychological, and Clinical Information.
| FESz, Mean (SD) | HC, Mean (SD) | P | |
|---|---|---|---|
| Age, years | 22.6 (5.1) | 21.1 (3.0) | .18 |
| Gender, male/female, n | 19/8 | 18/9 | .77 |
| SES | 31.9 (13.2) | 33.7 (13.6) | .61 |
| Parental SES | 43.1 (12.6) | 47.5 (13.5) | .23 |
| WASI IQ | 110.4 (14.5) | 108.0 (10.7) | .48 |
| MATRICS | 42.5 (13.3) | 47.2 (8.2) | .13 |
| PANSS Total | 75.2 (14.8) | ||
| PANSS Positive | 20.0 (5.6) | ||
| PANSS Negative | 16.9 (4.9) | ||
| PANSS General | 38.3 (7.5) | ||
| PANSS TD | 11.2 (3.1) | ||
| SAPS Global | 5.9 (2.9) | ||
| SANS Global | 9.7 (3.1) | ||
| Illness duration,a days | 73.3 (120.4) | ||
| DUP,b days | 192.2 (201.1) | ||
| Medicationc | 228.8 (147.9) |
Abbreviations: FESz, first-episode schizophrenia; HC, healthy controls; SES, socioeconomic status; WASI, Wechsler Abbreviated Scale of Intelligence; MATRICS, composite scaled t score; PANSS, Positive and Negative Syndrome Scale; TD, PANSS Thought Disorder factor; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; DUP, duration of untreated psychosis.
Illness duration indicates the full psychosis duration (in days).
DUP indicates the duration of untreated psychosis, time since first psychotic symptom until treatment or testing (in days).
Medication indicates chlorpromazine equivalents.
Diagnostic Assessments
Diagnosis was based on the Structured Clinical Interview for DSM-IV (SCID-P). Symptoms were rated using the Positive and Negative Syndrome Scale (PANSS), Scale for Assessment of Positive Symptoms (SAPS), and Scale for Assessment of Negative Symptoms (SANS).
Neuropsychological Functioning
All participants completed the MATRICS Cognitive Consensus Battery and the WASI.
EEG
EEG was recorded from a custom 72-channel Active2 high impedance system (BioSemi), comprising 70 10–10 scalp sites including the mastoids, 1 nose electrode, and 1 electrode below the right eye. The EEG amplifier bandpass was DC to 104 Hz (24 dB/octave roll off) digitized at 512 Hz, referenced to a common mode sense site (near PO1).
MMN Procedure
Auditory stimuli were presented while EEG was recorded and participants watched a silent video. Stimuli comprised a standard tone (1 kHZ, 50 ms duration, 5 ms rise/fall, 80 dB), a pitch deviant (1.2 kHZ, 50 ms duration, 5 ms rise/fall, 80 dB), and a duration deviant (1 kHZ, 100 ms duration, 5 ms rise/fall, 80 dB), presented with a stimulus onset asynchrony of 330 ms. A total of 1600 tones were presented, including 1280 standards (80%), 160 pitch deviants (10%) and 160 duration deviants (10%). Due to time constraints, a subset of 6 FESz and 7 HC participants were only presented a total of 800 tones, including 640 standards (80%), 80 pitch deviants (10%) and 80 duration deviants (10%).
MMN Measurement
Processing was done off-line with BESA (BESA GmbH) and BrainVision Analyzer2 (Brain Products GmbH). First, using BESA, EEG was filtered between 0.5 Hz (to remove DC drifts and skin potentials) and 20 Hz (to remove muscle and other high-frequency artifact). Data were visually examined and any channels with excessive noise were interpolated. Independent component analysis was used to remove 1 vertical and 1 horizontal EOG component. Next, in BrainVision Analyzer2, data were re-referenced to the nose tip. Epochs of 350 ms, including a 50-ms prestimulus baseline, were extracted for deviant tones and the standard tones preceding a deviant. Epochs were baseline corrected, and subsequently rejected if any site contained activity ±50 μV. Averages were constructed for the standard tones preceding a deviant (to equate signal to noise between all averages), pitch deviants, and duration deviants. pMMN was visualized by subtracting the standard average from the pitch deviant average. dMMN was visualized by subtracting the standard average from the duration deviant average. MMN was measured by automatic detection of the peak MMN at Fz (pMMN window: 90–145 ms; dMMN window: 140–200 ms). The number of accepted deviant trials did not differ between groups (pMMN—FESz: 122.1 ± 32.5, HC: 116.9 ± 30.9, P > .5; dMMN—FESz: 120.7 ± 32.8, HC: 116.4 ± 31.2, P > .6). Following visual verification and adjustment if needed, mean voltage over a 100-ms window (peak ±50 ms) was calculated. The Fz site was used for correlation to attempt to replicate the structural-functional (HG-MMN) association observed at that site in Salisbury et al,7 although we note the MMN paradigm and averaging procedures here differed.
Magnetic Resonance Imaging
The primary anatomical structure of interest was left HG, containing primary and portions of secondary auditory cortices. High-resolution T1-weighted structural MRI data (3 T) were acquired for each subject using a Siemens Tim Trio with a 32-channel phase array head coil. A magnetization-prepared rapid gradient echo sequence (MPRAGE) was acquired. MPRAGE parameters were repetition time (TR) = 2530 ms, inversion time (TI) = 1260 ms, multi-echo echo time (TE) (TE1 = 1.74 ms, TE2 = 3.6 ms, TE3 = 5.46 ms, TE4 = 7.32 ms), flip angle = 7°, voxel size = 1 × 1 × 1 mm, 176 slices. High-resolution spin echo: TR = 5040 ms, TE = 30 ms, 60 slices, 55° flip angle, field of view (FOV) = 220 × 220 × 138 mm. Freesurfer was used to segment white matter, gray matter, and pial surfaces on the structural MRI reconstruction. The boundary between gray and white matter in HG was hand-traced using the method of Barta et al.21 HG was identified as a prominent fold anterior to the planum temporale on the superior temporal lobe, beginning medially just caudal to the insula and terminating at the lateral edge of the temporal lobe where it meets the superior temporal gyrus.
In addition, the following criteria were applied:
Case 1. If there was more than one prominent gyrus running across the temporal lobe, the most anterior gyrus was taken to be HG.
Case 2. If there was one prominent transverse temporal gyrus with a smaller gyrus running anterior to it, the more prominent, posterior gyrus was labeled as HG.
Case 3. If the prominent transverse gyrus had a division such that it was difficult to tell which branch constituted the lateral end of HG, then the posterior branch was taken.
The same criteria were applied for both left and right HG measurement.
Freesurfer was used to calculate the values of gray matter volume in left and right HG. Absolute volumes were used for all correlations. For group comparisons of HG volumes, absolute volumes were normalized by intracranial volume, and multiplied by 100 to express volumes as percent of cranial contents.
Analyses
Demographic and neuropsychological measures and MMN at Fz were compared between groups using a t-test. Sex distributions were compared using Pearson’s chi-square. HG volumes were compared between groups using repeated-measures analysis of variance, with one between-groups factor of diagnosis (2 levels) and one within-groups factor of hemisphere (2 levels). Correlations were assessed with Spearman’s rho. Significance was set at P ≤ .05.
Results
Demographic and Neuropsychological Measures
Groups did not differ in age, socioeconomic status (SES), parental SES, WASI IQ, or MATRICS, or in sex distributions (Table 1).
MMN
pMMN and dMMN waveforms are presented in Figure 1. Groups did not differ in peak pMMN latency (FESz: 113.1 ± 13.8 ms; HC: 117.8 ± 14.5 ms, t52 = 1.22, P = .23). pMMN was significantly reduced in this sample of FESz (FESz: 2.0 ± 1.4 μV; HC: 3.1 ± 1.7 μV, t52 = 2.50, P = .016). Groups did not differ in peak dMMN latency (FESz: 174.3 ± 14.7 ms; HC: 173.9 ± 20.1 ms, t52 = 0.08, P = .94). dMMN amplitude was not reduced in FESz (1.8 ± 1.5 μV; HC: 2.1 ± 1.6 μV, t52 = 0.77, P = .44).
Figure 1.
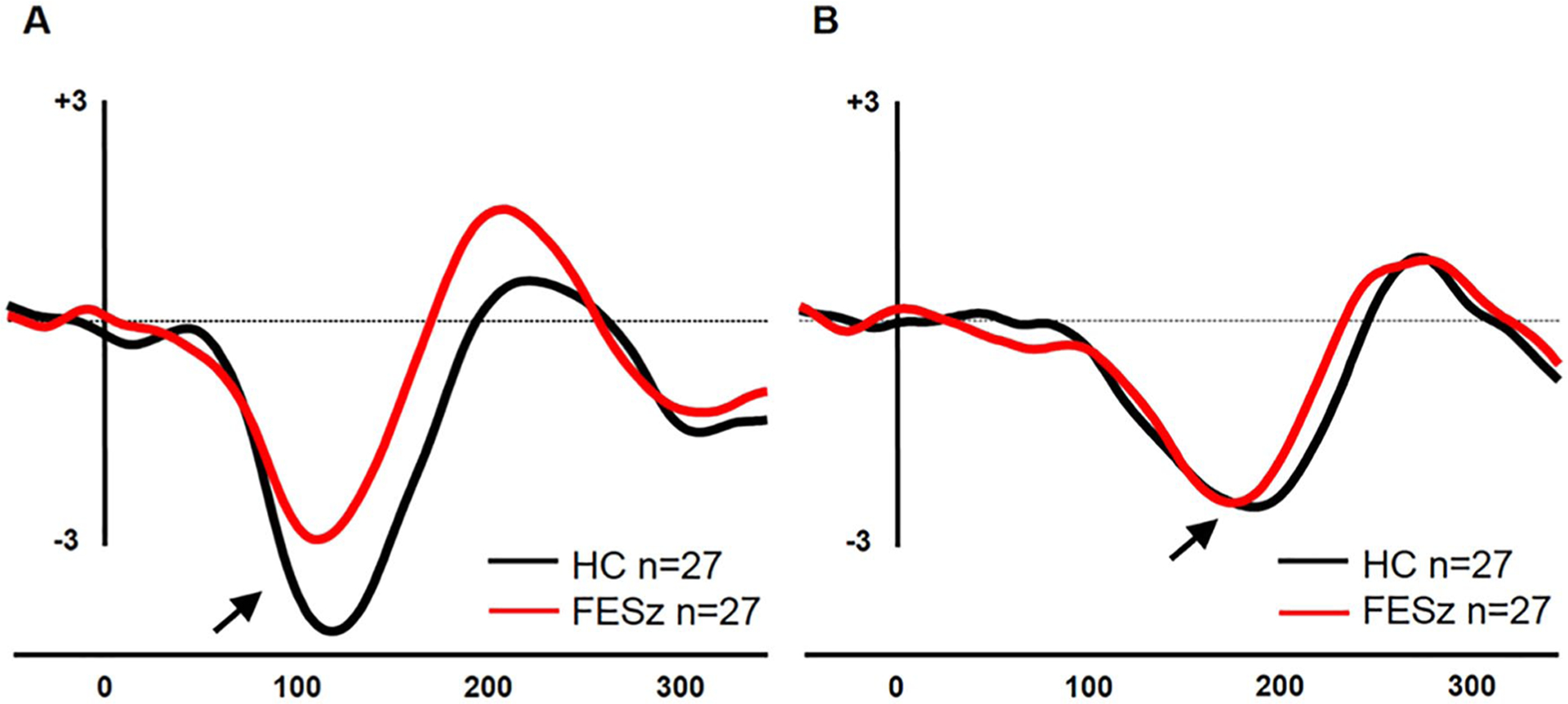
Group mismatch negativity (MMN) grand averages at Fz. (A) Pitch-deviant MMN (pMMN) subtraction waveforms. (B) Duration-deviant MMN (dMMN) subtraction waveforms.
HG
HG parcellation is indicated in Figure 2. Absolute and relative gray matter volumes are presented in Table 2. Groups did not differ significantly in total relative HG gray matter volumes (F[1, 52] = 0.04, P = .84). Left hemisphere HG gray matter was larger than right (F[1, 52] = 28.19, P < .001), and this asymmetry did not differ between groups (F[1, 52] = 0.30, P = .59).
Figure 2.
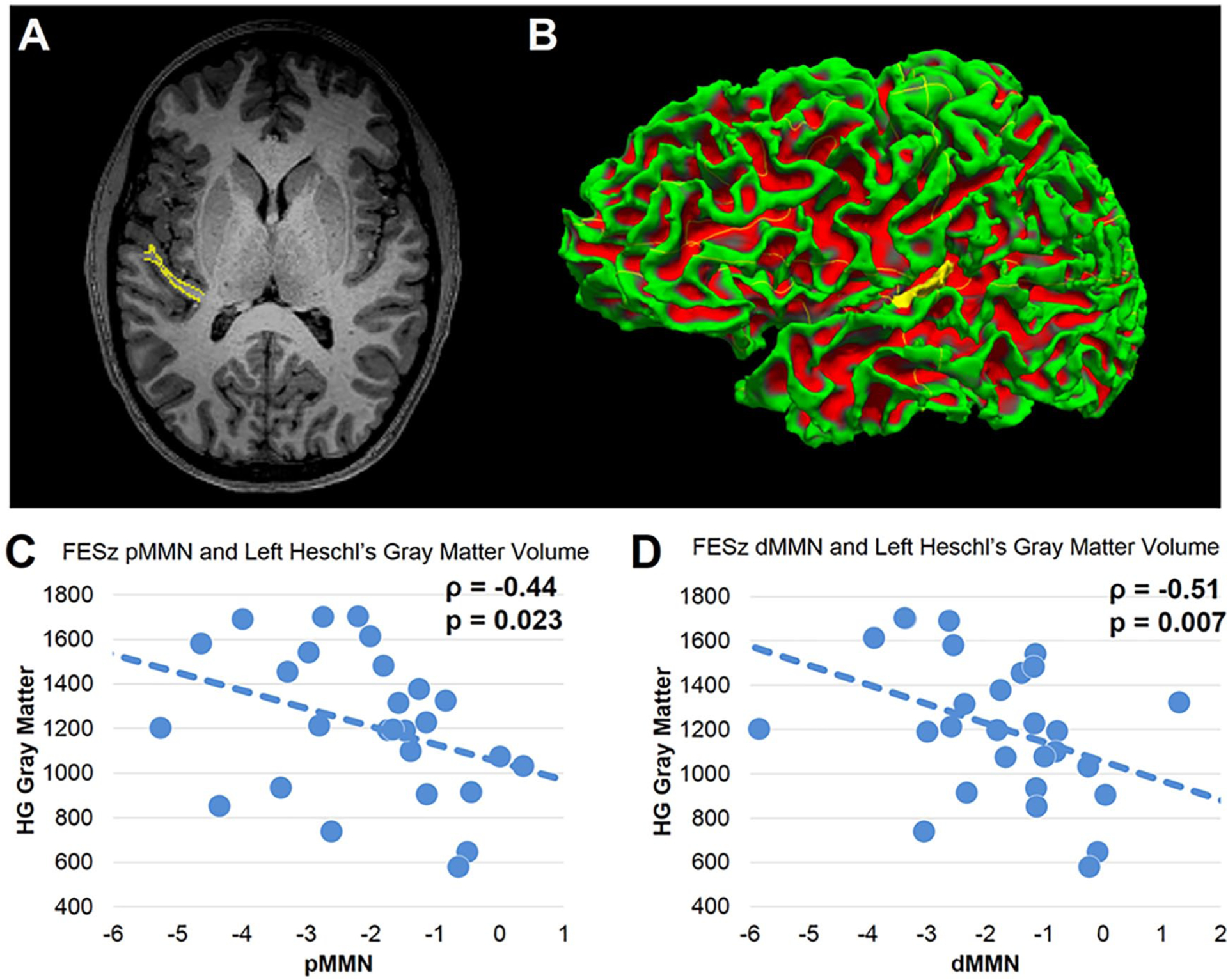
(A) Magnetic resonance imaging (MRI) slice tracing of Heschl’s gyrus (HG). (B) Reconstruction of HG. (C) Correlation between pitch-dominant mismatch negativity (pMMN) at Fz and left HG gray matter volume in first-episode schizophrenia (FESz). (D) Correlation between duration-dominant mismatch negativity (dMMN) at Fz and left HG gray matter volume in FESz.
Table 2.
HG volume measures.
| FESz | HC | P | |
|---|---|---|---|
| Left HG absolute GMV | 1213.4 (321.8) | 1245.3 (260.7) | .69 |
| Right HG absolute GMV | 986.4 (203.6) | 1046.1 (162.2) | .24 |
| ICV | 1599928.0 (207945.1) | 1620355.6 (210393.3) | .72 |
| Left HG relative GMVa | 0.08 (0.03) | 0.08 (0.02) | .95 |
| Right HG relative GMVa | 0.06 (0.02) | 0.07 (0.01) | .60 |
Abbreviations: HG, Heschl’s gyrus; GMV, gray matter volume; ICV, intracranial volume.
Relative GMV is percent ICV.
Association Between MMNs and Heschl’s Gyrus Gray Matter Volumes
Within FESz, pMMN correlated with left HG gray matter volume (ρ = −0.44, P = .023), but not right hemisphere HG gray matter volume (ρ = −0.09, P = .65). No association for left or right HG was present in HC. The dMMN-left HG correlation was significantly different in FESz and HC (Fisher’s Z = 2.3, P = .02).
dMMN correlated with left HG gray matter volume in FESz (ρ = −0.51, P = .007), and at trend-level with right hemisphere HG gray matter volume (ρ = −0.36, P = .07). No association for left or right HG was present in HC. The dMMN-left HG correlation was significantly different in FESz and HC (Fisher’s Z = 2.0, P = .046) but not for right HG (Fisher’s Z = 1.1, P = .26). Scattergrams of MMN values and HG gray matter volumes in FESz are presented in Figure 2.
Discussion
Our primary finding was that both pMMN and dMMN correlate with left hemisphere HG gray matter volumes at initial clinical contact for psychosis in the schizophrenia-spectrum. The pMMN association is consistent with the finding of Salisbury et al7 in first hospitalized individuals, but extends this to a sample even earlier in disease course, at first contact for clinical services. The dMMN association is novel, and suggests that the processes underlying timing deviance detection are also impaired by the left hemisphere early gray matter loss thought to precede overt psychosis.7,18,19 While the pMMN-HG gray matter association was not present for right hemisphere, there was a trend-level association between dMMN and right HG gray matter volumes. This finding suggests that dMMN may, in fact be more sensitive to the underlying pathology of schizophrenia, as proposed by Michie.20 However, it should be noted that the dMMN - right HG correlation in FESz was not statistically different from HC, and thus should be interpreted with caution. Together, these results suggest that in a subset of patients there is enough gray matter loss prior to the onset of psychosis to affect functional measures of brain activity, although subtle enough not to manifest as an overall group gray matter loss. These relationships being more marked in the left hemisphere are consistent with studies showing preferential gray matter loss in left temporal areas in FESz.18,19
Within this FESz sample, pMMN was significantly reduced, whereas dMMN was not. This is contrary to the larger body of literature in FESz, where pMMN reductions have generally not been reported. When present in FESz, reductions are typically seen in dMMN. It is unclear what might account for this discrepancy. Inspection of Figure 1 suggests that the HC show a rather large pMMN, and in comparison with our larger HC samples, the current HC sample comprises large pMMN amplitudes. Currently we are testing a larger independent sample of FESZ to determine whether this pMMN reduction was an anomaly or is present reliably early in disease course, and of HC to determine whether this sample simply had large pMMN.
We do not have longitudinal follow-up in these individuals, and thus cannot determine if MMN reductions and HG reductions occur, or the extent to which such reductions (if present) correlate. Currently, we are testing an independent cohort at first clinical contact and 3, 6, and 12 months later to determine the longitudinal course of structural and functional changes.
Several additional caveats must be mentioned. The samples, though large for FESz studies, are relatively modest. Thus, replication in independent samples is crucial to determine if the effects are present across individuals. This is the second demonstration of the pMMN–left HG association, and thus likely reflects a stable association. However, the broad interval (100 ms) and restriction to Fz do not allow for precise decomposition of precisely what areas and subprocesses for MMN are involved. Work utilizing MEG and source-reconstruction, and fine grained cortical parcellation including functional areas such as A1 would greatly improve the translation to actual cortical systems. If successful, such localization might identify key areas for targeted intervention using noninvasive brain stimulation.
In summary, pMMN and dMMN correlated strongly with left hemisphere HG gray matter volumes in FESz in the context of overall group gray matter volumes within normal limits. This association is present very early in disease course, proximal to first clinical contact for psychosis. This finding is consistent with a progressive cortical neuropil reduction during the prodromal stage of psychosis that precedes the emergence of overt psychosis. pMMN and dMMN are sensitive indices of this reduction. To that extent, they serve as biomarkers of disease progression, and appear to be excellent candidate outcome measures for interventions. If pMMN or dMMN are utilized as treatment outcomes, interventions that keep MMN large, whether noninvasive brain stimulation, sensory/cognitive training, or pharmacologic, can be inferred to interfere with the pathological gray matter loss associated with psychosis proximal to onset.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIMH R01 MH094328 (DFS, Principal Investigator) and P50 MH103204 (DFS, Project Co-Principal Investigator). We thank the faculty and staff of the WPIC Psychosis Recruitment and Assessment Core, the Conte Center for Translational Mental Health Research (P50 MH103204, David Lewis, MD, Director), and the University of Pittsburgh Clinical Translational Science Institute (UL1 RR024153, Steven E. Reis, MD) for their assistance in recruitment, diagnostic and psychopathological assessments, and neuropsychological evaluations.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dierks T, Linden D, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. [DOI] [PubMed] [Google Scholar]
- 2.Tiihonen J, Hari R, Naukkarinen H, Rimón R, Jousmäki V, Kajola M. Modified activity of the human auditory cortex during auditory hallucinations. Am J Psychiatry. 1992;149:255–257. [DOI] [PubMed] [Google Scholar]
- 3.Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. [DOI] [PubMed] [Google Scholar]
- 4.Edgar JC, Hanlon FM, Huang MX, et al. Superior temporal gyrus spectral abnormalities in schizophrenia. Psychophysiology. 2008;45:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford JM, Roach BJ, Palzes VA, Mathalon DH. Using concurrent EEG and fMRI to probe the state of the brain in schizophrenia. Neuroimage Clin. 2016;12:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgar JC, Hunter MA, Huang M, et al. Temporal and frontal cortical thickness associations with M100 auditory activity and attention in healthy controls and individuals with schizophrenia. Schizophr Res. 2012;140:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. [DOI] [PubMed] [Google Scholar]
- 9.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. [DOI] [PubMed] [Google Scholar]
- 10.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haigh SM, Coffman BA, Salisbury DF. Mismatch negativity in first-episode schizophrenia: a meta-analysis. Clin EEG Neurosci. 2017;48:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devrim-Üçok M, Keskin-Ergen HY, Üçok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:179–185. [DOI] [PubMed] [Google Scholar]
- 13.Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW. Pitch and duration mismatch negativity and pre-morbid intellect in the first hospitalized schizophrenia spectrum. Schizophr Bull. 2017;43:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Näätänen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topogr. 1995;7:315–320. [DOI] [PubMed] [Google Scholar]
- 15.Hari R, Hämäläinen M, Ilmoniemi R, et al. Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man. Neurosci Lett. 1984;50:127–132. [DOI] [PubMed] [Google Scholar]
- 16.Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. [DOI] [PubMed] [Google Scholar]
- 17.Kropotov JD, Alho K, Näätänen R, et al. Human auditory-cortex mechanisms of preattentive sound discrimination. Neurosci Lett. 2000;280:87–90. [DOI] [PubMed] [Google Scholar]
- 18.Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42:177–194. [DOI] [PubMed] [Google Scholar]
- 21.Barta PE, Petty RG, McGilchrist I, et al. Asymmetry of the planum temporale: methodological considerations and clinical associations. Psychiatry Res. 1995;61:137–150. [DOI] [PubMed] [Google Scholar]


