Fig. 2. Physiological but disease-enabling functions of tau.
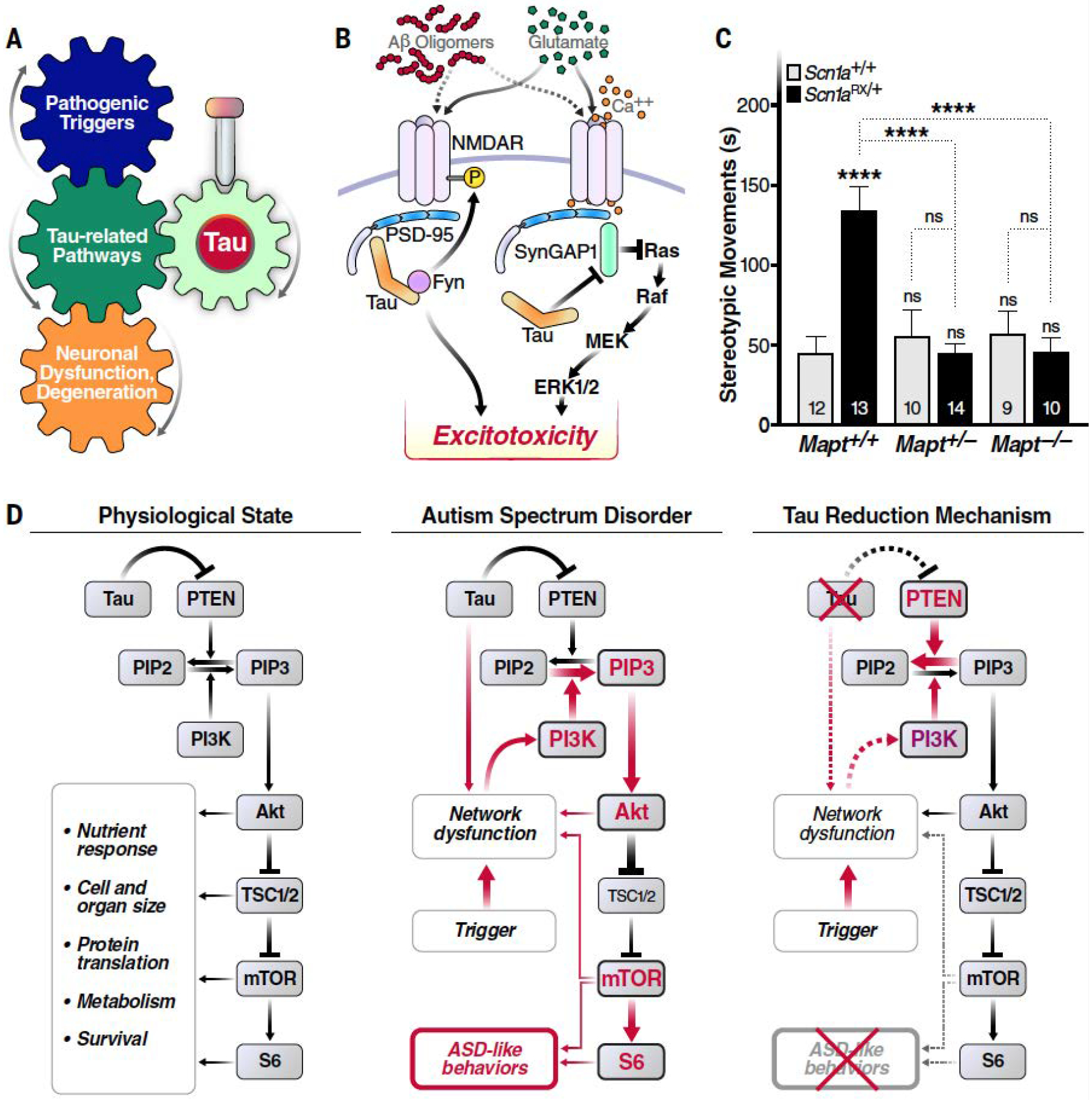
(A) Simplified conceptual diagram highlighting how targeting tau could block the development or progression of disease, even when tau is neither a driver nor a direct mediator of the pathogenic cascade. (B) Tau regulates interactions among the tyrosine kinase Fyn, N-methyl-D aspartate receptors (NMDARs), and postsynaptic density protein 95 (PSD-95) (44), as well as between PSD-95 and synaptic Ras GTPase-activating protein 1 (SynGAP1), which negatively regulates synaptic extracellular signal-regulated kinase (ERK) activation (20). Although these tau activities may normally form part of the complex network of regulators that support synaptic and neuronal activities, they may also allow pathological overstimulation of glutamate receptors to result in “excitotoxicity,” a process tau reduction has been shown to counteract (4, 20). P, phosphorylation; MEK, mitogen-activated protein kinase kinase. [Adapted from figure 3 of (4) with permission from Elsevier] (C and D) In mouse models, the presence of endogenous WT tau allows other processes to overactivate the PI3K-Akt-mTOR signaling pathway, which in turn promotes the development of autism-like behaviors, including excessive stereotypic movements, social interaction deficits and cognitive inflexibility (18). (C) Partial or complete genetic reduction of tau prevented excessive stereotypic movements (time spent engaged in such movements in seconds) in Scn1aRX/+ knockin mice, which carry a sodium channel mutation that causes autism and epilepsy in humans. By contrast, tau reduction did not alter this behavior in mice without this mutation. Numbers of mice per group are indicated in bars. ****P < 0.0001 vs. WT (Scn1a+/+Mapt+/+) controls or as indicated by brackets [two-way analysis of variance (ANOVA) with Holm-Sidak test]. ns, not significant. Error bars indicate SEM. [Reprinted from figure 1A of (18) with permission from Elsevier] (D) Because PTEN counteracts PI3K, tau’s ability to inhibit PTEN activation (46) may enable normal activity of the PI3K-Akt-mTOR pathway under physiological conditions. However, when pathological processes overactivate PI3K, as in some autism spectrum disorders, tau’s constraint of PTEN becomes maladaptive and allows for overactivation of the downstream signaling cascade, promoting the development of autism symptoms through diverse anatomical and pathophysiological mechanisms. Tau reduction counteracts this process by releasing the activity of PTEN and possibly also by blocking neural network dysfunctions. PIP2, phosphatidylinositol 4,5-biphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; TSC1/2, tuberous sclerosis 1 and 2; S6, ribosomal protein S6; ASD, autism spectrum disorder. [Reprinted from figure 3 of (18) with permission from Elsevier]
