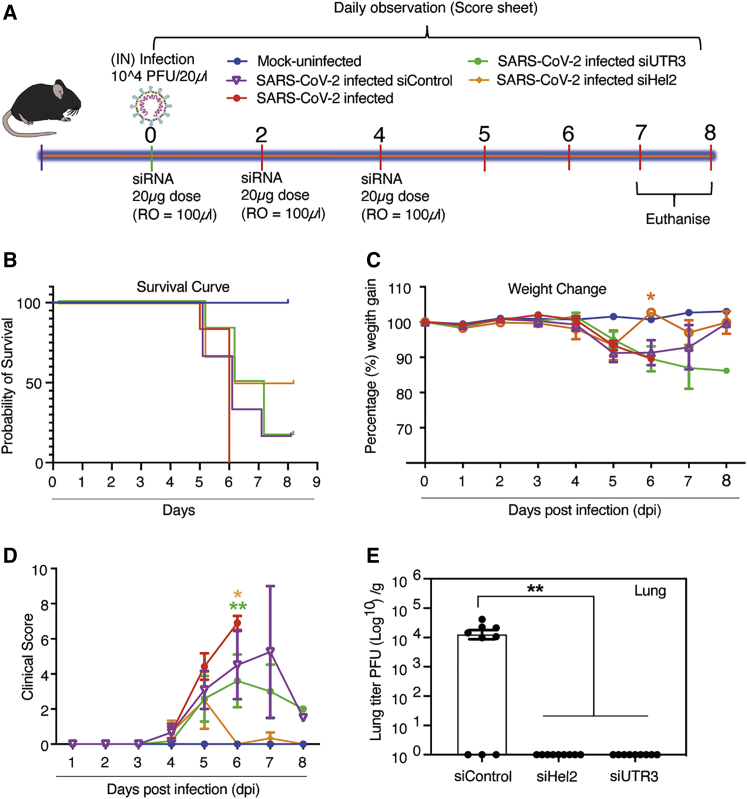
Figure 4.
Intravenous administered dmLNP-siRNA suppression of COVID-19 in vivo
(A) 7- to 13-week-old K18-hACE2 female and male mice were intranasally infected with either PBS or 1 × 104 PFU/20 μL of SARS-CoV-2 (Australian VIC1 strain, passage 4). (A) Mice (n = 6 for each treatment arm) were i.v. treated with 1 mg/kg in 100 μL of siRNA packaged into DOTAP 40 LNPs by retro-orbital administration at 0, 2, and 4 dpi. At 6–8 dpi, lung tissues were harvested and homogenized for immunoplaque assays. (B–D) Mouse survivorship during infection and dmLNP-siRNA treatment, (B) probability of survival, (C) body weight (weight change), and (D) clinical score were evaluated at the indicated dpi. Mice that lost >15% of their initial body weight were humanely euthanized and plotted as a non-survivor. (B–D) Mice were weighed and scored daily until the experimental endpoint for disease progression. (D) The clinical score was evaluated based on locomotion, behavior, and appearance. Each data point represents the average ± SEM of 3 to 4 mice. (E) The amount of infectious virus particles in lung tissues at 6–8 dpi (n = 3 mice) was determined by immunoplaque assays on Vero E6 cells, using a SARS-CoV-2 N protein-specific antibody and expressed as PFU per gram of tissue. Each data point represents a technical replicate, where one mouse is equivalent to 3 technical replicates and bars represent the average ± SEM p < 0.0517 and ∗∗p < 0.01 are considered statistically significant when assessed by two-way ANOVA (Dunnett’s post-test) against31 SARS-CoV-2 infected only mice and (E) siControl.