Abstract
BACKGROUND AND PURPOSE:Symptomatic intracranial stenoses have a high risk for a recurrent stroke if treated medically. Although angioplasty and stent placement are proposed treatment options, data on longer-term outcome are limited.
Materials and METHODS:We analyzed all endovascular procedures on symptomatic intracranial stenosis at our institution from January 1998 to December 2005. We retrospectively assigned patients to group A (symptoms despite antithrombotic therapy) or group B (impaired regional cerebral blood flow [rCBF]). Primary outcome events were periprocedural major complications or recurrent ischemic strokes in the territory of the treated artery. We used the Kaplan-Meier method to calculate survival probabilities.
RESULTS:The procedural technical success rate was 92% (35/38) with periprocedural major complications in 4 cases (10.5%; group A [8.3%, 2/24], group B [14.3%, 2/14]). Median (range) follow-up for the 33 patients with technically successful procedures was 21 (0–72) months. Recurrent ischemic strokes occurred in 15% (3/20) of patients in group A and 0% (0/13) of patients in group B. Overall, there were 21% (7/33) primary outcome events (group A [25%, 5/20], group B [15%, 2/13]). There was a nonsignificant trend for better longer-term survival free of a major complication or recurrent stroke in patients with impaired rCBF compared with patients who were refractory to medical therapy treatment (Kaplan-Meier estimate 0.85 [SE 0.10] vs 0.72 [SE 0.11] at 2 years, respectively).
CONCLUSION:Interventional treatment of symptomatic intracranial stenosis carries significant risk for complications and recurrent stroke in high-risk patients. The observation that patients with impaired rCBF may have greater longer-term benefit than medically refractory deserves further study.
Atherosclerotic stenosis of the major intracranial arteries accounts for 8%–10% of all ischemic strokes1, 2 and, after a symptomatic event, the annual risk of recurrence is high (estimates, 8%–11%).3–5 However, the optimum strategy for secondary prevention in this situation remains uncertain. In particular, the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study failed to show a benefit of warfarin compared with aspirin in the prevention of recurrent ischemic events.4
Interventional procedures such as percutaneous transluminal angioplasty (PTA) and stent placement have been proposed as potentially more effective methods of secondary prevention. Two groups of patients are frequently considered as potential target groups for endovascular therapy. First, patients who have recurrent ischemic events despite standard medical therapy and second (and more recently), patients with clinically apparent impaired regional cerebral blood flow (rCBF) secondary to the stenoses.6–9 Both groups are considered to be at especially high risk for stroke, which justifies the use of a potentially risky procedure. However, the acute risks and long-term outcome of such endovascular therapies in both subgroups are still poorly described.
The purpose of this study, therefore, was to report our experience with angioplasty and stent placement for symptomatic intracranial atherosclerotic stenoses in patients who have either failed medical therapy or who have impaired rCBF secondary to intracranial stenoses.
Patients and Techniques
Patients
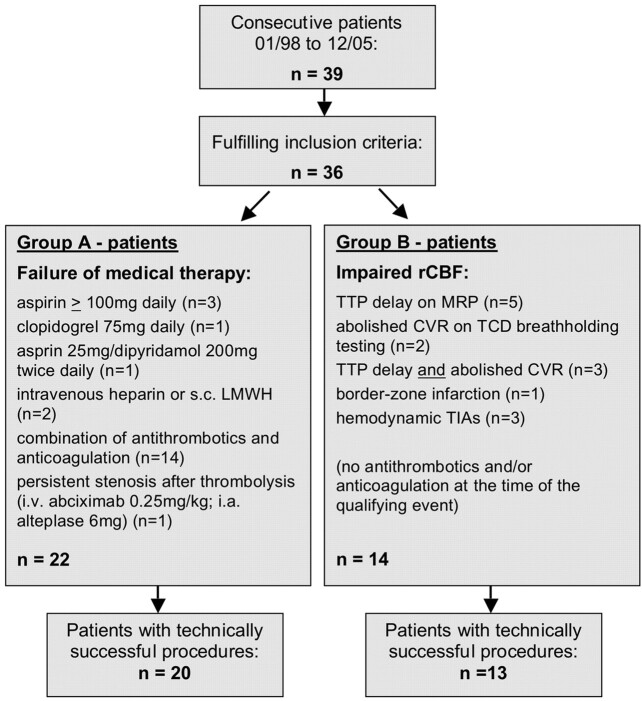
We retrospectively reviewed the medical records of consecutive patients who underwent angioplasty or stent placement (angioplasty with stent placement or primary stent placement) of intracranial stenoses between January 1998 and December 2005 at our institution. We identified patients by using a data base maintained by the Department of Neuroradiology and analyzed only those with symptomatic intracranial atherosclerotic stenoses. Patients with nonatherosclerotic, extracranial, or asymptomatic stenoses were excluded. Endovascular revascularization was offered if the treating physicians (neuroradiologist and neurologist) concluded that a given patient was at high risk for an imminent ischemic stroke. We retrospectively assigned patients to 1 of 2 groups:
Group A: Patients with failure of medical therapy defined as a transient ischemic attack (TIA) or ischemic stroke attributable to the index intracranial stenosis despite treatment with antithrombotic medications or the persistence of severe stenosis after thrombolytic treatment for acute ischemic stroke;
Group B: Patients with symptomatic intracranial stenosis associated with impaired rCBF as defined by 1) time-to-peak (TTP) delay on MR perfusion imaging (MRP), 2) abolished cerebrovascular reserve capacity (CVR) on transcranial Doppler (TCD) breath-hold testing, 3) borderzone infarction, or 4) blood pressure-dependent fluctuation of neurologic symptoms. Patients in this group were not receiving antithrombotic treatment at the time of their first symptomatic event (Fig 1).
In each case, we assessed the National Institutes of Health Stroke Scale (NIHSS) score within 2 hours before and after each procedure. MR imaging including MR angiography (MRA), diffusion-weighted (DWI) and perfusion imaging (MRP), and complete sonographic examination was performed 24 hours or less before and after the procedure in most patients. CT was performed if MR imaging was not feasible (before procedure [n = 6] or after procedure [n = 7]). Preprocedural CVR with TCD breath-hold testing was determined in 9 patients with the use of previously described criteria.10
Fig 1.
Characteristics of groups A and B.
Procedure
All procedures were performed by 1 of 3 experienced interventional neuroradiologists (D.M., A.M., R.v.K.) with the patients under local anesthesia or, in patients with decreased levels of consciousness or agitation, general anesthesia. Before the procedure, the patients received various combinations of antiplatelet agents or anticoagulation, or both. During the procedure, intravenous heparin (bolus 3000–5000 IE) was given to all patients to maintain systemic anticoagulation with a target activated clotting time of 250 to 300 seconds. The decision to use additional agents (intravenous [IV] abciximab; intra-arterial [IA] urokinase) was made on a case-by-case basis by the treating physicians.
A 6F introducer sheath was placed into the femoral artery with the use of percutaneous access and a 6F Envoy guiding catheter (Cordis, Miami Lakes, Fla) advanced to the distal cervical internal carotid (ICA) or vertebral artery (VA). A selected balloon catheter or stent delivery device was advanced over a Transcend-14 guidewire (Boston Scientific, Natick, Mass) and navigated to the site of the stenosis. The decision to perform angioplasty, angioplasty with stent placement, or primary stent placement and the selection of devices was left to the discretion of the treating interventionalist. In general, primary stent placement was attempted if considered feasible on the basis of assessment of tortuosity of the proximal vessel and morphology of the lesion from the beginning of this series until May 2005, when a report suggested beneficial long-term clinical outcome after angioplasty.11, 12 Since then, we aimed for angioplasty and performed subsequent stent placement only if the stenosis was felt to have not improved after angioplasty or if a dissection after angioplasty was felt to require treatment.
Balloons (FasStealth, Maverick; Boston Scientific) and balloon-expandable stents (INX, Cerebrence; Medtronic AVE, Santa Rosa, Calif; TAXUS, Liberté; Boston Scientific) were selected to provide submaximum inflation to avoid dissection or rupture of the vessel. We did not use self-expanding stents in this study. Repeated procedures were performed at the discretion of the interventionalist. After stent-assisted angioplasties, the combination of aspirin and clopidogrel was given for at least 4 weeks. After PTA alone, antithrombotic agents were given at the discretion of the treating physician.
Follow-Up
We followed our patients at regular visits (1, 3, 6, and 12 months, then yearly) to our neurovascular laboratory (Department of Neurology). Each visit included a clinical and sonographic examination of the target vessel. Elective MRA was performed in 1 patient with insufficient transtemporal sonographic approach 3 months after the procedure. We performed repeat angiograms only if clinical or sonographic data suggested restenosis. If a follow-up visit was not feasible, we evaluated the patient’s recent clinical status by telephone interview (n = 9).
Outcome Measures
We measured a number of outcomes:
Technical success: For analysis of clinical outcomes in patients with technically successful procedures, we defined technical success as completion of angioplasty, angioplasty with stent placement, or primary stent placement with even a slight decrease in the degree of stenosis. In addition, we categorized percentages of residual stenosis into less than 50%, 50% to 69%, and 70% or more according to a recent report by Marks.12 We estimated the degree of stenosis by comparing the diameter of the vessel at the site of the stenosis (D stenosis) with the normal diameter of the vessel just distal to the stenosis (D distal) using the formula: % stenosis = [1 − (D stenosis/D distal)] x 100% according to the criteria of the North American Symptomatic Carotid Endarterectomy Trial (NASCET).13 When severe preocclusive stenosis made it too difficult to perform an accurate measurement, we reported the stenoses as 99%.
Improvement of perfusion parameters: visual decrease in the extent of contrast inflow delay on TTP maps on MRP as stated in the expert report given the same technique and parameters were used before and after the intervention, normalization of CVR on TCD breath-hold testing, or clinical stabilization in patients with clinical hemodynamic characteristics;
Major complications: any stroke resulting in deterioration of the NIHSS score of 3 points or more or death within 30 days after the procedure;
Minor complications: any stroke resulting in deterioration of the NIHSS score of 2 points or less within 30 days after the procedure;
Asymptomatic complications: procedure-related vessel dissection or asymptomatic hemorrhagic infarct transformation without change in NIHSS score;
Recurrent stroke and TIA: acute onset of new focal neurologic symptoms attributable to the index intracranial stenosis lasting greater (stroke) or less (TIA) than 24 hours and occurring more than 30 days after a successful procedure;
Restenosis: peak systolic Doppler-flow velocity greater than or equal to pretreatment velocity, repeat angiography with degree of stenosis greater than or equal to pretreatment degree, severe stenosis on repeat MRA, or target vessel occlusion proved on angiogram (DSA, CTA, MRA).
All patients with recurrent clinical events received repeat CT or MR imaging. Major periprocedural and recurrent strokes were subclassified as fatal if deemed to have caused the death of the patient at any stage after the stroke, disabling if the patient had a modified Rankin scale (mRS) score of more than 2, and nondisabling if the patient had an mRS score of 2 or less at 3 months.
Our primary outcome measure was survival without a major complication or recurrent ischemic stroke in patients who had a technically successful procedure. We chose this compound outcome to estimate the efficacy of completed intracranial angioplasty or stent placement.
Data Analysis
We used the unpaired t test for comparison of means, the Mann-Whitney U test for comparison of medians, the Fisher exact test for comparison of proportions, and Kaplan-Meier curves to calculate survival free of a primary outcome event. When patients experienced more than 1 primary outcome event, we based the survival analysis on the outcome event that occurred first. We compared survival curves using the logrank test. For all analyses, we took P < .05 as statistically significant. We used the SPSS statistical package (version 12.0) for these analyses (SPSS, Chicago, Ill).
Results
Patients
For 96 months, 39 patients underwent 41 interventional procedures. Three patients were excluded because their stenoses were asymptomatic (n = 2) or nonatherosclerotic (n = 1). One additional patient underwent 2 repeat procedures for symptomatic restenosis of the originally treated lesion. This report, therefore, relates to 38 procedures in 36 patients (28 men, mean ± standard deviation (SD) age 58 ± 12 years). All patients had 50% or more intracranial stenoses by NASCET criteria. Median interval from the qualifying event to the procedure was 9.5 days (range, 0–201 days). We performed 8 (21%) procedures acutely with equal percentages in groups A and B in patients who presented with fluctuation of clinical symptoms (n = 2), progressive stroke (n = 5), or recurrent ischemic stroke directly before the procedure (n = 1). Baseline characteristics are summarized in detail in Table 1.
Table 1:
Baseline characteristics
| Variable | All (n = 38 Procedures in 36 Patients) | Group A (n = 24 Procedures in 22 Patients) | Group B (n = 14 Procedures in 14 Patients) | P Value (Group A vs Group B) |
|---|---|---|---|---|
| Age (mean ± SD) | 58 ± 12 | 60 ± 12 | 55 ± 12 | .26 |
| Men, n (%) | 28/36 (78) | 16/22 (73) | 12/14 (86) | .44 |
| Known risk factors, n (%) | ||||
| Arterial HTN | 34/36 (94) | 22/22 (100) | 12/14 (86) | .14 |
| Hypercholesterolemia | 25/36 (69) | 16/22 (73) | 9/14 (64) | .72 |
| Diabetes | 14/36 (39) | 8/22 (36) | 6/14 (43) | .74 |
| Coronary artery disease | 8/36 (22) | 8/22 (36) | 0/14 (0) | .01 |
| Smoking (any time) | 6/36 (17) | 2/22 (9) | 4/14 (29) | .18 |
| Peripheral vascular disease | 3/36 (8) | 3/22 (14) | 0/14 (0) | .27 |
| Degree of stenosis ( mean ± SD) | 80 ± 14 | 82 ± 14 | 77 ± 13 | .37 |
| Location of stenosis, n (%) | .175 | |||
| Anterior circulation | 24/38 (63) | 13/24 (54) | 11/14 (79) | |
| Posterior circulation | 14/38 (37) | 11/24 (46) | 3/14 (21) | |
| Qualifying event, n (%) | .50 | |||
| TIA | 14/38 | 10/24 (42) | 4/14 (29) | |
| Ischemic stroke | 24/38 | 14/24 (58) | 10/14 (71) | |
| Time since event (days), median (range) | 9.5 (0–201) | 9 (0–117) | 24 (0–201) | .19 |
| Acute intervention, n (%) | 8/38 (21) | 5/24 (21) | 3/14 (21) | 1.00 |
Note:—SD indicates standard deviation; HTN, hypertension; TIA, transient ischemic attack.
The indication to perform interventional therapy was medical failure for 22 (group A) and impaired rCBF for 14 patients (group B). Thirteen patients in group A also had criteria indicating impaired rCBF. Compared with group A, the patients in group B tended to be younger, to have stenosis in the anterior rather than posterior circulation, to have an ischemic stroke rather than a TIA as the qualifying event, to be treated with greater delay, and to have concomitant coronary artery disease (Table 1).
Procedures and Immediate Results
Three procedures for stenoses in the posterior circulation (2 in group A, 1 in group B) were technically unsuccessful because of tortuosity of the proximal vessel (Table 2). None of these procedures caused major or minor complications. Angioplasty alone (n = 12), angioplasty with stent placement (n = 3), or primary stent placement (n = 20) could be performed in 92% (35/38). Percentages of angioplasty and stent placement (angioplasty with stent placement or primary stent placement) were similarly distributed between groups A and B (Table 2). Characteristics of the procedure for each patient are summarized in Table 3.
Table 2:
Procedural outcomes
| All Patients (n = 38 Procedures) | Group A Patients (n = 24 Procedures) | Group B Patients (n = 14 Procedures) | P Value (Group A vs Group B) | |
|---|---|---|---|---|
| Type of intervention, n (%) | .88 | |||
| PTA | 12/38 (32) | 7/24 (29) | 5/14 (36) | |
| Stent | 23/38 (60) | 15/24 (63) | 8/14 (57) | |
| Not successful | 3/38 (8) | 2/24 (8) | 1/14 (7) | |
| Degree of stenosis (mean ± SD) | ||||
| Before procedure | 80 ± 14 | 82 ± 14 | 77 ± 13 | .37 |
| After procedure | 24 ± 26 | 21 ± 25 | 30 ± 27 | .33 |
| Major complication, n (%) | 4/38 (10.5) | 2/24 (8.3) | 2/14 (14.3) | .62 |
| Restenosis, n (%) | 10/29 (35) | 6/17 (35) | 4/12 (33) | 1.00 |
Note:—PTA indicates percutaneous transluminal angioplasty; SD, standard deviation.
Table 3:
Summary of all procedures
| No./Age/Sex | Indication | Lesion Site | Procedure | Stent Type | Concomitant Medication | Stenosis Pre/Post | Follow-Up Modality/Duration | Restenosis |
|---|---|---|---|---|---|---|---|---|
| 1/69/M | ↓ rCBF | Left VA | PTA | − | − | 95/55 | TCD/88 months | No |
| 2/51/F | ↓ rCBF | Right ICA | PTA | − | − | 85/70 | TCD/72 months | No |
| 3/69/M | Medical failure | BA | PTA | − | − | 80/50 | TCD/36 months | Yes |
| 4/61/M | ↓ rCBF | Right ICA | Primary stenting | AVE | IV abciximab (bolus 20 mg) | 60/0 | TCD/68 months | No |
| 5a/64/M | Medical failure | Left MCA | PTA | − | − | 64/39 | TCD/7 months (confirmed DSA) | Yes |
| 5b | Medical failure | PTA | − | IV abciximab (bolus 10 mg) | 77/0 | TCD/36 months (confirmed MRA, DSA) | Yes | |
| 5c | Medical failure | Primary stenting | Liberté | − | 90/0 | TCD/3 months | No | |
| 6/53/F | Medical failure | Left MCA | PTA | − | − | 73/68 | TCD/49 months | No |
| 7/60/M | ↓ rCBF | Left ICA | PTA & stenting | INX | − | 83/0 | TCD/45 months | No |
| 8/73/M | Medical failure | Right MCA | Primary stenting | INX | IV abciximab (bolus 10 mg) | 99/50 | − | − |
| 9/76/M | Medical failure | Left ICA | Primary stenting | INX | − | 83/0 | − | − |
| 10/47/F | Medical failure | Left MCA | PTA | − | − | 99/17 | TCD/7 months (confirmed MRA) | Yes |
| 11/29/M | Medical failure | Left MCA | PTA & stenting | INX | IA urokinase (0.5 MIU/1 hour) | 57/0 | TCD/40 months | No |
| 12/45/M | ↓ rCBF | Left MCA | Primary stenting | INX | IV abciximab (bolus 10 mg) | 75/50 | TCD/2 months (confirmed DSA) | Yes |
| 13/59/M | Medical failure | BA | Primary stenting | INX | IV abciximab (bolus 10 mg) | 99/23 | TCD/22 months | No |
| 14/41/M | ↓ rCBF | Left MCA | Primary stenting | Cerebrence | − | 74/10 | TCD/25 months | No |
| 15/51/M | ↓ rCBF | Right ICA | Primary stenting | INX | − | 75/0 | TCD/7 months (confirmed MRA) | Yes |
| 16/65/M | ↓ rCBF | Left ICA | Primary stenting | INX | − | 67/50 | TCD/24 months | Yes |
| 17/63/M | Medical failure | Left ICA | Primary stenting | Cerebrence | − | 65/0 | TCD/1 month | No |
| 18/69/M | Medical failure | Left VA | Primary stenting | Liberté | − | 99/0 | TCD/28 months | No |
| 19/37/M | Medical failure | Right MCA | Primary stenting | TAXUS | IV abciximab (bolus 20 mg) | 90/0 | TCD/2.5 months (confirmed MRA, DSA) | Yes (in-stent thrombosis) |
| 20/66/F | Medical failure | Left MCA | Primary stenting | TAXUS | − | 90/0 | TCD/4 months | No |
| 21/57/F | Medical failure | Left ICA | Primary stenting | Liberté | − | 80/60 | − | − |
| 22/73/F | Medical failure | BA | n.s. | − | IV abciximab (bolus 5 mg) | 99 | − | − |
| 23/57/M | Medical failure | BA | n.s. | − | IV abciximab (bolus 20 mg) | 99 | − | − |
| 24/72/F | Medical failure | Left MCA | Primary stenting | TAXUS | − | 70/0 | MRA/3 months | No |
| 25/61/M | Medical failure | Right VA | Primary stenting | Liberté | − | 99/10 | − | − |
| 26/54/M | Medical failure | BA | Primary stenting | TAXUS | − | 99/10 | TCD/13 months | No |
| 27/63/M | Medical failure | BA | Primary stenting | Liberté | IV abciximab (bolus 20 mg, infusion 7.2 mg/12 hours) IA tPA (6 mg) | 82/0 | TCD/1 month | Yes |
| 28/41/M | ↓ rCBF | Right MCA | Primary stenting | TAXUS | − | 90/0 | TCD/18 months | No |
| 29/67/M | ↓ rCBF | Left ICA | Primary stenting | Liberté | − | 80/60 | TCD/3 days (confirmed MRA) | Yes (in-stent thrombosis) |
| 30/42/M | Medical failure | BA | PTA | − | − | 80/60 | − | − |
| 31/74/M | ↓ rCBF | Right MCA | PTA | − | IV abciximab (bolus 17.5 mg, infusion 6.3 mg/12 hours) | 72/30 | TCD/12 months | No |
| 32/69/M | Medical failure | Left VA | PTA | − | − | 70/40 | TCD/9 months | No |
| 33/66/M | ↓ rCBF | BA | n.s. | − | IV abciximab (bolus 20 mg, infusion 7.2 mg/12 hours) | 99 | − | − |
| 34/41/F | ↓ rCBF | Right MCA | PTA | − | − | 50/25 | TCD/10 months | No |
| 35/41/M | ↓ rCBF | BA | PTA | − | IV abciximab (bolus 20 mg, infusion 7.2 mg/12 hours) | 90/50 | − | − |
| 36/66/M | Medical failure | BA | PTA & stenting | Liberté | − | 61/25 | TCD/10 months | No |
Note:—↓ rCBF indicates impaired regional cerebral blood flow; n.s., not successful; PTA, percutaneous transluminal angioplasty; MCA, middle cerebral artery; ICA, internal carotid artery; VA, vertebral artery; BA, basilar artery; MRA, MR angiography; DSA, digital subtraction angiography; TCD, transcranial Doppler; IA, intra-arterial; IV, intravenous; tPA, tissue plasminogen activator.
Abciximab was given at varying dosages in 12 procedures (7/24 in group A, 5/14 in group B). The indication was to prevent periprocedural formation of a thrombus in patients with a severely irregular vascular surface at the site of the stenosis in 11 cases. In 1 procedure (patient 27), IV abciximab was given as part of an institutional protocol in combination with IA alteplase (6 mg) for basilar artery thrombosis. We performed subsequent primary stent placement for persistent severe basilar artery stenoses. One patient (patient 11) with periprocedural embolic angular gyrus artery occlusion was treated successfully with IA thrombolysis (0.5 MIU urokinase; bolus 0.1 MIU, infusion 0.4 MIU for 1 hour). The patient did not have a persistent neurologic deficit.
Overall, the completed procedures resulted in an immediate reduction in the degree of stenosis (mean ± SD) from 80% ± 14% to 24% ± 26% without major differences between groups A and B. Residual stenosis was less than 50% in 24 (69%) procedures, 50% to 69% in 10 (29%) procedures, and 70% or more in 1 (3%) procedure.
Technically successful procedures in patients in group B or group A with additional signs of impaired rCBF resulted in improvement or normalization of perfusion parameters in 91% (20/22) with available postprocedural assessment (improvement [n = 6], normalization [n = 5] or conversion to hyperperfusion [n = 2] of previous TTP delay on MRP, normalization of CVR on TCD breath-hold testing [n = 4], and clinical stabilization in patients with clinical hemodynamic characteristics [n = 3]).
Complications
Major complications occurred during 4 (10.5%) of 38 procedures (Table 2). Two patients suffered a reperfusion injury with symptomatic intracranial hemorrhage (ICH) occurring 60 and 90 minutes after successful procedures. One was fatal in a patient (patient 8) who received periprocedural abciximab. The other patient (patient 28) was functionally independent at 3 months. Two patients had periprocedural ischemic strokes in the territory of the treated artery, 1 of which was fatal at 5 weeks in patient 21. The other patient (patient 29) suffered in-stent thrombosis 3 days after stent placement provoked by unintentional discontinuance of clopidogrel. He remained functionally dependent at 3 months.
The major complication rate tended to be higher in patients in group B compared with those in group A (2/14 [14.3%] vs 2/24 [8.3%], respectively), stent placement compared with angioplasty (4/23 [17.4%] vs 0/12 [0.0%], respectively), and stenoses in the anterior compared with the posterior circulation (4/24 [16.7%] vs 0/14 [0.0%], respectively). None of these differences were statistically significant. There was no difference in the major complication rate for procedures performed earlier or later than the median delay since the qualifying event (9.5 days; both 10.5%).
Minor complications occurred in 4 (10.5%) of 38 procedures, all of them minor embolic strokes and none with permanent neurologic sequelae (temporary expressive aphasia, hypesthesia of the right foot, memory deficits, vertigo, and diplopia). Asymptomatic complications occurred in 6 of 38 procedures (proximal vessel dissections [n = 4] treated with an extracranial carotid stent in 1 patient, asymptomatic DWI lesions on postinterventional MR imaging [n = 2], and asymptomatic hemorrhagic infarct transformation [n = 1]).
Recurrent Stroke and TIA
We assessed recurrent events in the 33 patients (20 in group A, 13 in group B) who had technically successful procedures. Median follow-up was 21 months (range, 0–72 months) for the entire cohort, 16 months (range, 0–49 months) for patients in group A, and 30 months (range, 0–72 months) for patients in group B (Table 4). Overall, 3 patients had recurrent ischemic strokes, all in group A. One stroke was disabling in a patient (patient 9) with restenosis of the extracranial ICA after combined primary stent placement of tandem extracranial and intracranial stenosis of the ICA. Two recurrent strokes were nondisabling. The cause was restenosis of the MCA 7 months after angioplasty in 1 patient (patient 5) and in-stent thrombosis 2.5 months after stent-assisted angioplasty with a drug-eluting stent in another patient (patient 19). In the latter, clopidogrel had been discontinued for a kidney biopsy. Recurrent TIAs occurred in 1 patient (patient 3) in group A with basilar artery restenosis 36 months after angioplasty. There were no recurrent events in the patients from group B.
Table 4:
Clinical outcome of patients with technically successful procedures
| All Patients (n = 33) | Group A (n = 20) | Group B (n = 13) | P Value (Group A vs Group B) | |
|---|---|---|---|---|
| Follow-up (mos), median (range) | 21 (0–72) | 16 (0–49) | 30 (0–72) | .15 |
| Recurrent ischemic stroke, n (%) | 3 (9) | 3 (15) | 0 (0) | .26 |
| Recurrent TIA, n (%) | 1 (3) | 1 (5) | 0 (0) | 1.00 |
| Primary outcome events (major complication or recurrent ischemic stroke), n (%) | 7 (21.2) | 5 (25.0) | 2 (15.4) | .68 |
| Survival without primary outcome event | .51 | |||
| Probability at 1 year (SE) | 0.81 (0.07) | 0.79 (0.10) | 0.85 (0.10) | |
| Probability at 2 years (SE) | 0.77 (0.08) | 0.72 (0.11) | 0.85 (0.10) |
Note:—TIA indicates transient ischemic attack; SE, standard error.
Primary Outcome Measure
A primary clinical outcome event occurred in 7 (21.2%) of 33 patients with technically successful procedures (4 major complications, 3 recurrent ischemic strokes). The Kaplan-Meier estimate for survival without a primary outcome event at 2 years was 0.77 (standard error [SE] 0.08; Fig 2A).
Fig 2.
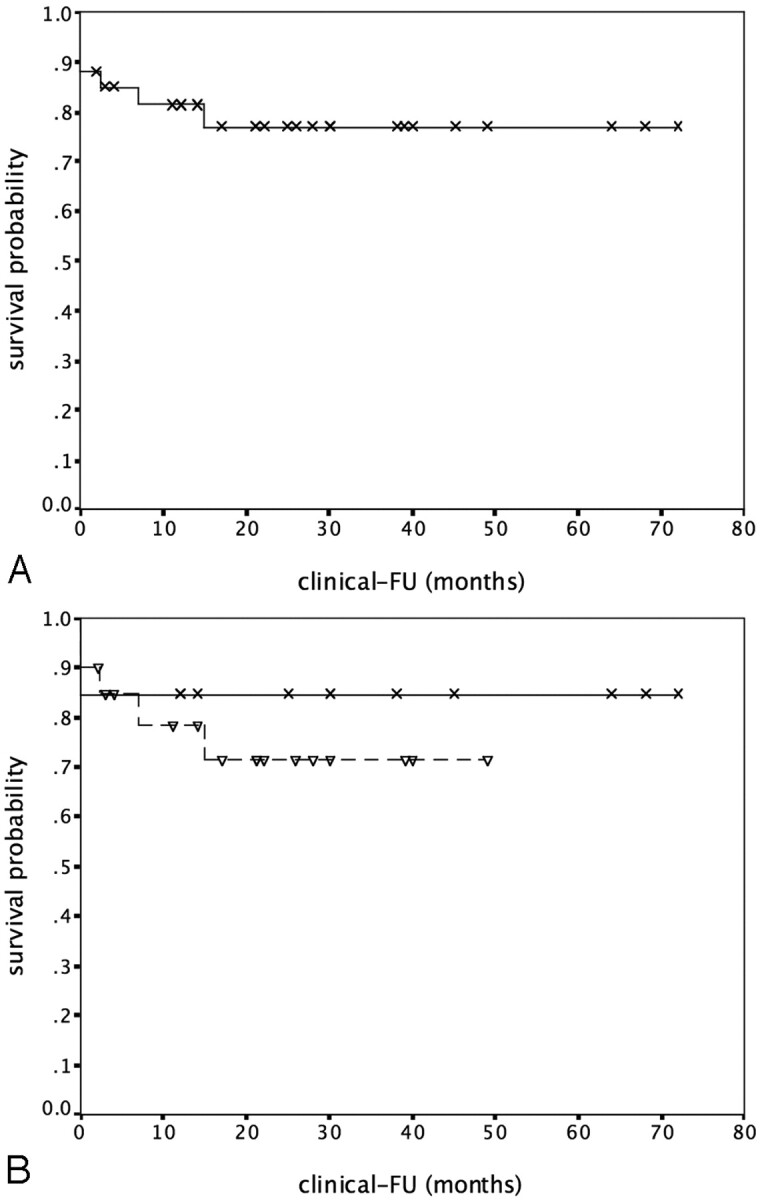
Kaplan-Meier curves for probability of survival without a major complication or recurrent stroke (FU indicates follow-up). 2A, All patients with a successful intervention. 2B, Patients with failure of medical treatment (dashed line) or impaired rCBF (solid line; logrank test, P = .51).
Primary outcome events occurred in 5 (25%) of 20 patients of group A (2 major complications, 3 recurrent strokes) and 2 (15%) of 13 patients of group B (2 major complications). Kaplan-Meier estimates for survival without a major complication or recurrent stroke at 2 years were 0.72 (SE, 0.11) for patients in group A and 0.85 (SE, 0.10) for patients in group B (logrank test, P = .51; Fig 2B).
If stratified by procedures, primary outcome events occurred in 1 (9%) of 11 patients treated with angioplasty (1 recurrent stroke) compared with 6 (27%) of 22 patients treated with stent placement (4 major complications, 2 recurrent strokes). Kaplan-Meier estimates for survival without a primary outcome event at 2 years were 0.90 (SE, 0.09) for patients treated with angioplasty compared with 0.72 (SE, 0.10) for those treated with stent placement (logrank test, P = .26).
Restenosis
Data on restenosis refer to 29 procedures in which follow-up data were available. Median follow-up was 12 months (range, 0–88 months). The overall rate of restenosis was 35% (10/29) with median time to restenosis of 7 months. Rates of restenosis were 35% (6/17) for group A and 33% (4/12) for group B (Table 2). If stratified by procedures, rates of restenosis were 40% (4/10) for angioplasty and 32% (6/19) for stent placement. Overall, 5 (50%) of 10 restenoses were symptomatic.
Discussion
During 21 months of median follow-up after angioplasty or stent placement for symptomatic intracranial atherosclerotic stenosis, the overall rate of major complications or recurrent ipsilateral ischemic stroke was 21.2% in our study. We observed a nonsignificant trend toward better long-term survival free of stroke in patients with impaired rCBF (group B) compared with patients who were refractory to medical treatment (group A). Two-year probabilities for survival without a major complication or recurrent stroke were 0.85 (group B) versus 0.72 (group A), respectively. No patient with impaired rCBF had a recurrent ischemic event after a successful intervention.
The overall rate of periprocedural complications or recurrent stroke seems high compared with an overall 2-year stroke rate in the territory of the treated artery of 14% in the Wafarin-Aspirin Symptomatic Intracranial Disease (WASID) population.5 However, defining subgroups with a higher risk for recurrence if treated medically is relevant when determining the potential benefit of interventional treatment. Although often (including our study) considered a target population for interventional treatment, patients with antithrombotic therapy at the time of the qualifying event did not have higher risk for stroke recurrence in the WASID trial.5, 14 In contrast, a more severe stenosis indicated a higher annual risk for stroke (0.07 [50%–59% stenosis] to 0.31 [80%–89% stenosis]), thus suggesting that cerebral hypoperfusion is a contributing pathophysiologic factor.15 In fact, patients with clinically significant hemodynamic intracranial stenoses had a nearly 2-fold risk for recurrence compared with patients without this finding in a recent study (60.7% vs 31.7% at 23.4 months, respectively).16
We observed postprocedural improvement or normalization of previous pathologic perfusion parameters in 91%. Patients who had not failed medical therapy but had stenoses resulting in impaired rCBF did not have recurrent ipsilesional ischemic events after a successful intervention. Thus, angioplasty and stent placement may have been an effective treatment for this condition. In contrast, 3 recurrent ischemic strokes and 1 recurrent TIA occurred in patients who had failed medical therapy, which suggests that these patients may have more severe atherosclerotic disease with potentially less benefit from interventional treatment.17
Current data on longer-term outcome after intracranial angioplasty and stent placement are inconclusive.18 Recent studies report cumulative 2-year stroke rates of 8.2% (primary stent placement) to 11.8% (angioplasty) compared with 21.2% in our study.12, 19 However, these studies excluded patients who were treated acutely, and interventional treatment was delayed up to 6 weeks after a symptomatic event. Because time since the qualifying event independently predicted the risk for stroke recurrence in the WASID trial with the highest risk if enrolled within 17 days after a symptomatic event, interventional treatment may particularly allow benefit if performed early, as shown for extracranial carotid disease.5, 20 We performed 8 procedures acutely. Median delay was 9.5 days compared with 30 days in the study by Jiang.19 One of the patients treated acutely suffered a fatal reperfusion hemorrhage, thus paralleling reports on acute extracranial carotid stent placement.21 However, procedures performed earlier or later than the median delay had the same major complication rate (10.5%).
The rate of periprocedural stroke and death was 4.5% to 6.6% in recent trials compared with 10.5% in our study.12, 19, 22, 23 Apart from timing, further issues need to be addressed:
Reported rates of neurologic complications vary greatly between 0% and 28%.24, 25 The overall rate of perioperative stroke and death for intracranial angioplasty and stent placement was 9.5% (95% CI, 7.0%–12.0%) in a recent Cochrane systematic review.18 Predictors for increased periprocedural risk have not been identified.
The patients in our study had criteria suggesting high risk for stroke. A recent study with similar patient characteristics (ie, failure of antithrombotic therapy plus evidence of perfusion failure) reported a major complication rate of 50%.14 Hemorrhagic complications in this study were partly attributed to the periprocedural application of abciximab in 5 patients, 4 of whom had a major hemorrhage (3 ICH). We applied abciximab at varying dosages within 12 procedures and observed reperfusion trauma with fatal ICH within 1. Because the association of abciximab with ICH during neurointerventional procedures is reported, choice of concomitant medication may be crucial.26
Intracranial stent placement might be more hazardous than angioplasty, given recently reported complication rates of 23.1% and 28.6%.27, 28 In our study, 23 of 35 technically successful procedures involved stent placement (angioplasty with stent placement or primary stent placement). All major complications occurred within these procedures. Newly designed self-expanding or balloon-expandable stents and increased level of training with high numbers of procedures performed may result in lower rates of complications.19, 23, 29 However, a benefit of intracranial stent placement compared with angioplasty on long-term clinical outcome has currently not been shown.
In our study, the clinical outcome tended to be better in patients who were treated with angioplasty compared with those who were treated with stent placement. This finding predominantly resulted from the higher complication rate with stent placement and may have skewed our data on long-term outcome. However, percentages of patients treated with stent placement versus those treated with angioplasty (and also occurrence of in-stent thromboses from discontinuation of antiplatelets) were nearly equally distributed within subgroups of patients with impaired rCBF and failure of medical treatment.
Our rate of restenosis of 35% is similar to 32.4% reported in the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) trial.22 Also similar to SSYLVIA, only 50% of restenoses were symptomatic, thus challenging their clinical significance. Nonetheless, all but 1 recurrent ischemic event was associated with restenoses. It is important to note that 2 patients had in-stent thrombosis after discontinuation of antiplatelet treatment, 1 occurring 2.5 months after primary stent placement with a drug-eluting stent. This finding underlines the need for dual antiplatelet therapy in the acute phase and for at least 12 months with drug-eluting stents as recommended in cardiology. Given cardiologists’ uncertainty, these stents should probably not be used for intracranial stent placement.30
Our study was hypothesis generating. The design of the study was retrospective, and treatment decisions were not based on a standardized protocol. Given small numbers, we reported trends rather than statistically significant findings. Both regional impairment of cerebral blood flow and failure of medical therapy were retrospectively defined by various criteria. In particular, visual assessment of TTP delay on MRP may have limited clinical significance and reproducibility. However, visual assessment of PWI sequences with TTP maps showed excellent interobserver agreement in patients with acute ischemic stroke.31 The application of NASCET criteria may have resulted in inaccurate measurement of stenosis in patients with long-segment stenoses or stenoses extending into bifurcations and may also allow limited comparison to studies that apply the WASID criteria.32 Furthermore, our definition of technical success is less rigorous compared with less than 50% or less than or equal to 30% residual stenosis applied in other studies.19, 22 We used this definition to analyze the clinical effect of angioplasty or stent placement with even slight reduction of stenosis. Future studies should address if greater stenosis reduction is associated with more favorable clinical outcomes. It is interesting to note that long-term clinical outcomes were favorable, though 40% of patients (compared with 31% in our study) had 50% or greater stenosis after angioplasty in a recent study by Marks and colleagues.12 Finally, because recruitment was achieved during an 8-year period, technical improvement and training may have influenced patient outcome.33
Conclusion
Angioplasty and stent placement for symptomatic intracranial atherosclerotic stenosis may have a significant periprocedural risk in patients with clinical or diagnostic criteria indicating high risk for a recurrent stroke. However, our data suggest that patients with intracranial stenosis resulting in impaired cerebral hemodynamics may have long-term benefits, whereas patients with failure of medical treatment may carry a significant risk despite a successful intervention.
References
- 1.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995;26:14–20 [DOI] [PubMed] [Google Scholar]
- 2.Wityk RJ, Lehman D, Klag M, et al. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke 1996;27:1974–80 [DOI] [PubMed] [Google Scholar]
- 3.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med 1985;313:1191–200 [DOI] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305–16 [DOI] [PubMed] [Google Scholar]
- 5.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555–63 [DOI] [PubMed] [Google Scholar]
- 6.Berkefeld J, Hamann GF, du Mesnil R, et al. [Endovascular treatment for intracranial stenoses. A common statement by neurologists and neuroradiologists]. Nervenarzt 2006;77:1444–55 [DOI] [PubMed] [Google Scholar]
- 7.Ecker RD, Levy EI, Sauvageau E, et al. Current concepts in the management of intracranial atherosclerotic disease. Neurosurgery 2006;59 (5 Suppl 3):S210–18; discussion S3–13 [DOI] [PubMed] [Google Scholar]
- 8.Higashida RT, Meyers PM. Intracranial angioplasty and stenting for cerebral atherosclerosis: new treatments for stroke are needed! Neuroradiology 2006;48:367–72 [DOI] [PubMed] [Google Scholar]
- 9.Klopfenstein JD, Ponce FA, Kim LJ, et al. Middle cerebral artery stenosis: endovascular and surgical options. Skull Base 2005;15:175–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett KM, Ackerman RH, Gahn G, et al. Basilar and middle cerebral artery reserve: a comparative study using transcranial Doppler and breath-holding techniques. Stroke 2001;32:2793–96 [DOI] [PubMed] [Google Scholar]
- 11.Marks MP, Wojak JC, Al-Ali F, et al. Angioplasty of intracranial stenosis: long-term clinical outcome. In Proceedings of the American Society of Neuroradiology (abstract);2005. .
- 12.Marks MP, Wojak JC, Al-Ali F, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke 2006;37:1016–20 [DOI] [PubMed] [Google Scholar]
- 13.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 14.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology 2000;55:490–97 [DOI] [PubMed] [Google Scholar]
- 15.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82 [DOI] [PubMed] [Google Scholar]
- 16.Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 2006;66:1187–91 [DOI] [PubMed] [Google Scholar]
- 17.Mori T, Fukuoka M, Kazita K, et al. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998;19:1525–33 [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Flores S, Diamond AL. Angioplasty for intracranial artery stenosis. Cochrane Database Syst Rev 2006;3:CD004133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang WJ, Xu XT, Du B, et al. Comparison of elective stenting of severe vs moderate intracranial atherosclerotic stenosis. Neurology 2007;68:420–26 [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–24 [DOI] [PubMed] [Google Scholar]
- 21.du Mesnil de Rochemont R, Sitzer M, Zanella FE, et al. [Stenting in acute stroke]. Radiologe 2005;45:461–65 [DOI] [PubMed] [Google Scholar]
- 22.SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke 2004;35:1388–92 [DOI] [PubMed] [Google Scholar]
- 23.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke 2007;38:881–87 [DOI] [PubMed] [Google Scholar]
- 24.Boulos AS, Agner C, Deshaies EM. Preliminary evidence supporting the safety of drug-eluting stents in neurovascular disease. Neurol Res 2005;27 Suppl 1:S95–102 [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Schumacher HC, Mangla S, et al. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology 2003;61:1729–35 [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Saad M, Zaidat OO, et al. Intracerebral hemorrhages associated with neurointerventional procedures using a combination of antithrombotic agents including abciximab. Stroke 2002;33:1916–19 [DOI] [PubMed] [Google Scholar]
- 27.Chow MM, Masaryk TJ, Woo HH, et al. Stent-assisted angioplasty of intracranial vertebrobasilar atherosclerosis: midterm analysis of clinical and radiologic predictors of neurological morbidity and mortality. AJNR Am J Neuroradiol 2005;26:869–74 [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JK, Ahn JY, Lee BH, et al. Elective stenting for symptomatic middle cerebral artery stenosis presenting as transient ischaemic deficits or stroke attacks: short term arteriographical and clinical outcome. J Neurol Neurosurg Psychiatry 2004;75:847–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang WJ, Xu XT, Jin M, et al. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. AJNR Am J Neuroradiol 2007;28:830–34 [PMC free article] [PubMed] [Google Scholar]
- 30.Maisel WH. Unanswered questions—drug-eluting stents and the risk of late thrombosis. N Engl J Med 2007;356:981–84 [DOI] [PubMed] [Google Scholar]
- 31.Girot M, Leclerc X, Gauvrit JY, et al. Cerebral magnetic resonance imaging within 6 hours of stroke onset: inter- and intra-observer reproducibility. Cerebrovasc Dis 2003;16:122–27 [DOI] [PubMed] [Google Scholar]
- 32.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–46 [PMC free article] [PubMed] [Google Scholar]
- 33.Sauvageau E, Ecker RD, Levy EI, et al. Recent advances in endoluminal revascularization for intracranial atherosclerotic disease. Neurol Res 2005;27 Suppl 1:S89–94 [DOI] [PubMed] [Google Scholar]