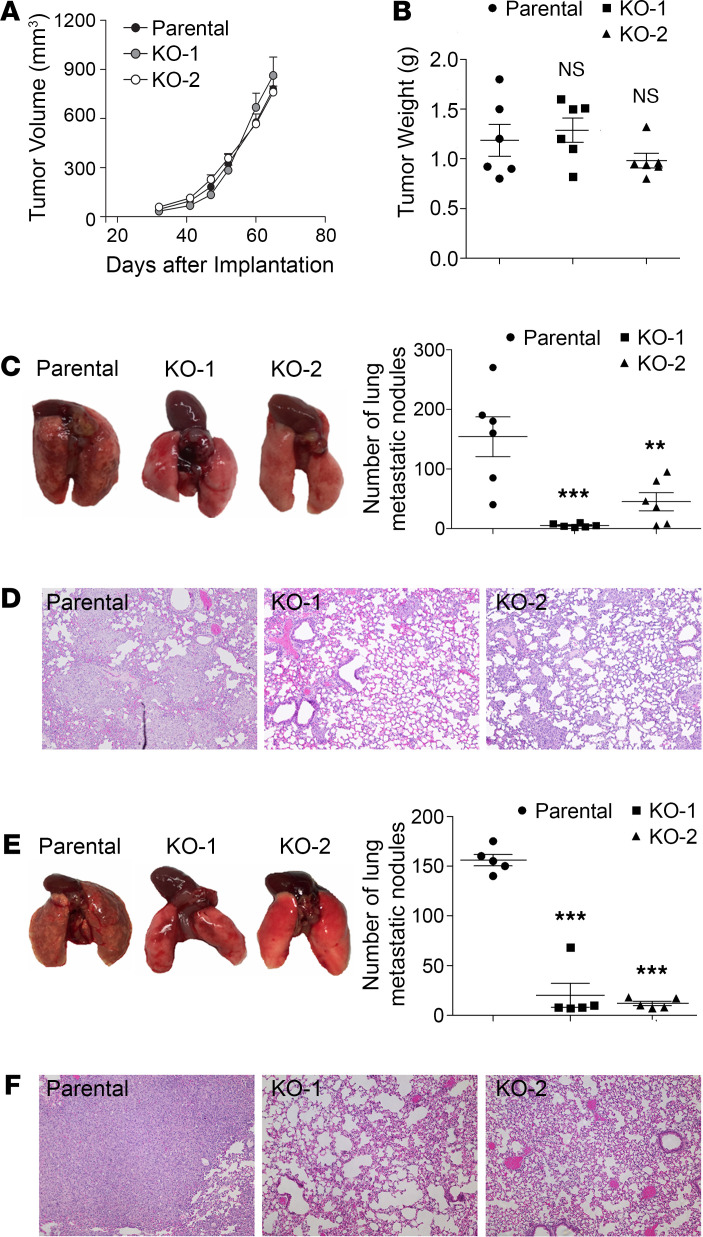
Figure 2. PD-L1 deficiency reduces the tumor metastasis independent of the antitumor immunity.
(A–D) Two million parental or PD-L1–null (KO-1 and KO-2) cells were injected into the mammary fat pad of NOD/SCID mouse (6 mice/group). (A) Tumor volume was measured with calipers weekly and calculated using the standard formula. (B) Tumors were dissected and weighed at the endpoint (65 days after inoculation). (C and D) Loss of PD-L1 inhibits lung metastasis. (C) Lungs were dissected at the endpoint. Left panel, images of gross lung showed that parental MDA-MB-231 tumors generated many more metastatic nodules on lung surface. (D) Representative H&E staining images of lung tissues showing micrometastatic lesions from mice bearing parental tumors are much more severe than those bearing PD-L1–null tumors. (E and F) Results from experimental metastasis model suggest that PD-L1 is necessary for the later steps of metastasis formation. Parental or PD-L1–null MDA-MB-231 cells (8 × 105) were directly injected into the tail vein of NOD/SCID mice (5 mice/group). Animals were terminated 40 days later to examine lung metastasis. (E) Metastatic nodules on lung surface in each group were analyzed. (F) Representative H&E staining images of lung tissues. PD-L1–null MDA-MB-231 tumors generated many fewer lung micrometastatic lesions than parental tumors. (D and F) Power of eyepiece: 10×; power of objective: 10×. (A–C and E) Data were plotted as mean ± SEM and statistically analyzed using 1-way ANOVA analysis with Dunnett’s test. n = 6 (A–C) and n = 5 (E). N.S., no significant difference; **, P < 0.01; ***, P < 0.001.