Abstract
Transcranial Magnetic Stimulation (TMS) is a non-invasive brain stimulation technique uniquely equipped to both examine and modulate neural systems and related cognitive and behavioral functions in humans. As an examination tool, TMS can be used in combination with electroencephalography (TMS-EEG) to elucidate directly, objectively, and non-invasively the intrinsic properties of a specific cortical region, including excitation, inhibition, reactivity, and oscillatory activity, irrespective of the participant’s conscious effort. Furthermore, when applied in repetitive patterns, TMS has been shown to modulate brain networks in healthy individuals, as well as ameliorate symptoms in individuals with psychiatric disorders. The key role of TMS in assessing and modulating neural dysfunctions and associated clinical and cognitive deficits in psychiatric populations is therefore becoming increasingly evident. Here, after a brief description of the origin of TMS, we review TMS-EEG studies in schizophrenia and mood disorders as most TMS-EEG studies to date focus on individuals with these disorders. We show evidence of repetitive TMS (rTMS) and Theta Burst Stimulation (TBS) targeting specific cortical areas in modulating neural circuits and ameliorating symptoms and abnormal behaviors in individuals with psychiatric disorders, especially when informed by resting state and task-related neuroimaging measures. We also provide examples of how the combination of TMS-EEG assessments and rTMS and TBS paradigms can be utilized to both characterize and modulate neural circuit alterations in individuals with psychiatric disorders. This approach, along with the evaluation of the behavioral effects of TMS-related neuromodulation, has potential to lead to the development of more effective and personalized interventions for individuals with psychiatric disorders.
Introduction
Noninvasive brain stimulation (NIBS) is a set of techniques that can be used to target brain circuits in vivo transcranially (1). Among NIBS techniques, Transcranial Magnetic Stimulation (TMS) is uniquely equipped to both examine and modulate neural systems and related cognitive and behavioral functions in humans (2).
As a probe, TMS can be used in combination with electroencephalogram (EEG) and with functional Magnetic Resonance Imaging (fMRI). TMS with concurrent fMRI presents several challenges, including the synchronization of TMS and fMRI signals, the effects of the magnetic field of the MR scanner on the TMS coil and the TMS-generated magnetic field, the difficulty of TMS coil positioning and brain targeting inside the scanner, the need to have access to a MR scanner and fMRI-compatible TMS coils, which makes it feasible only in a few specialized research centers (3, 4). In contrast, the availability of TMS with simultaneous EEG (TMS-EEG) has grown over the past several years. TMS-EEG offers the opportunity for investigating the activity and connectivity of neuronal circuits across various behavioral and pathophysiological states (5). TMS-EEG also gives certain advantages compared to traditional electrophysiological studies. First, EEG recordings collected using peripheral stimuli reach the cortical areas contributing to the scalp recorded signal after several synaptic relays and EEG measured during a task can be affected by participant motivation and level of cognitive engagement. TMS-EEG, however, can be used to elucidate directly, objectively, and non-invasively the intrinsic properties of a specific cortical region: excitation, inhibition, reactivity, and oscillatory activity, including its power, synchronization, and main oscillatory frequency, or natural frequency, irrespective of the participant’s conscious effort (6). This can help determine the neurophysiological properties of a given cortical area in healthy individuals as well as characterize how these properties may differ across different psychiatric disorders (7). Second, TMS-EEG can elucidate causal relationships between neural regions, i.e., the effect of one cortical area on the rest of the brain on a temporal scale that approximates neuronal activity (8). Hence, TMS-evoked EEG responses can be used to identify biological markers of brain health and disease as well as examine the functional integrity of neural circuits.
In addition to being utilized in combination with EEG as a probe, TMS can be applied in repetitive patterns to modulate brain networks in healthy individuals (9), as well as ameliorate symptoms in individuals affected by psychiatric disorders, especially major depressive disorder patients (10, 11). TMS can thus be used to induce acute changes in neural circuits while assessing, with EEG and cognitive tasks, the impact of these changes on neuronal and behavioral measures (12). This approach can, in turn, elucidate understanding of neural circuit-behavior relationships related to learning and memory (13, 14). A parallel approach, which pertains to psychiatric disorders, is to investigate more sustained effects of TMS on symptoms, which likely relies on the modulation of underlying neural circuits (15). The key role of TMS in assessing and modulating neural dysfunctions and associated clinical and cognitive deficits in psychiatric populations is therefore becoming increasingly evident (16).
In the following sections, we provide an overview of the different uses that TMS has in the examination of neural circuit dysfunction and neural circuit-behavioral relationships in psychiatric disorders. After a brief description of the origin of TMS, we review TMS-EEG studies in schizophrenia and mood disorders as most TMS-EEG studies to date focus on individuals with these disorders. Here, we show evidence of the efficacy of stimulation paradigms, including repetitive TMS (rTMS) and Theta Burst Stimulation (TBS), that target specific cortical areas in modulating neural circuits and in ameliorating symptoms and abnormal behaviors in individuals with psychiatric disorders especially when informed by resting state and task-related neuroimaging measures. We also provide some examples of how the combination of TMS-based assessments (i.e., TMS-EEG) and rTMS and TBS paradigms can be utilized to both characterize and modulate neural circuit alterations in individuals with psychiatric disorders. This approach, along with the evaluation of the behavioral effects of TMS-related neuromodulation, has potential to lead to the development of more effective interventions for individuals with psychiatric disorders.
The origin of TMS
TMS involves delivering brief, time-varying currents through insulated wires in an induction coil resting over the scalp. The resulting time-varying magnetic field, according to Faraday’s law, produces a secondary electrical current in underlying cortical neurons, whereas it does not usually reach deep brain structures. Although the exact neuronal substrate (i.e., axonal fibers vs. neuronal cell body or dendrites) has yet to be fully established, the TMS-induced electric field enhances neuronal cortical excitability and, if powerful enough, leads to neuronal discharge. Indeed, TMS was introduced by Anthony T. Baker in 1985 (17) as a tool to non-invasively investigate the functional properties of the motor corticospinal pathways in humans. Specifically, applying TMS to the motor cortex (e.g., hand motor area) can produce action potentials in a peripheral muscle (e.g., abductor pollicis brevis), which is described as motor-evoked potentials (MEPs). MEP amplitude, which depends on cortical, cortico-spinal, and spinal-muscular excitability, is a straightforward measure of corticospinal excitability, and, over the past three decades, various TMS/MEPs paradigms have been developed to assess excitation, inhibition, and plasticity of the motor cortex in both healthy individuals and individuals with psychiatric disorders (7, 18). For example, the resting motor threshold (RMT), which is considered a measure of motor corticospinal excitability, is the minimum TMS intensity needed to produce a MEP amplitude ≥50 μV in 5 out of 10 trials in a peripheral hand muscle at rest (18). Regarding motor cortical inhibition, short-interval intracortical inhibition (SICI) compares the MEP amplitude of a single, suprathreshold TMS test stimulus (TS) to a paired-pulse condition with a subthreshold conditioning stimulus (CS) followed by a suprathreshold TS after 2–5-ms, whereas long-interval cortical inhibition (LICI) compares a suprathreshold TS with a paired-pulse suprathreshold CS and TS at 50–200-ms intervals (19). Another measure of motor cortical inhibition, the silent period (SP), involves measuring the duration of absent muscle activity following a single, suprathreshold TS given during a muscle contraction. Furthermore, Intracortical facilitation (ICF) involves comparing a suprathreshold TS with a paired-pulse subthreshold CS and suprathreshold TS at 7–30-ms intervals (20).
Although TMS/MEPs paradigms have been extremely helpful in characterizing the neurophysiological properties of the motor cortex of healthy subjects as well as in identifying motor cortical abnormalities in psychiatric populations, as reviewed elsewhere (7, 19), these protocols could not provide direct information about other cortical areas.
TMS-EEG: the technique
The first demonstration of the feasibility of combining TMS with simultaneous EEG, was provided by Cracco and colleagues in 1989 by delivering TMS to the frontal cortex and measuring the EEG response in the contralateral homologous site (21). In that study, TMS evoked a contralateral positive EEG component with an onset latency of 8.8–12.2 msec, a duration of 7–15 msec, and an amplitude which reached up to 20 μV, thus providing initial evidence of human transcallosal responses to TMS. A few years later, Ilmoniemi et al. showed that TMS-EEG could be utilized to measure the local and long-distance cortical responses evoked by single pulse TMS of either motor or occipital areas (22). Building on this pioneering work, several TMS-EEG systems have been more recently developed to overcome the saturation of the EEG amplifiers due to the large TMS induced voltage that exceeds the 5mV voltage limit of conventional amplifiers. A sample-and-hold circuit, which involves blocking the input of the EEG amplifier from 50 μs prior to 2.5 ms after the TMS pulse while maintaining the voltage constant during this time interval, is an effective way to prevent the EEG amplifier saturation (23). A direct current (DC) amplifier, which combines a high sampling rate (e.g., >5 KHz) with a wide operational range (e.g., >5 mV), is also adequate since the amplifiers can absorb the TMS stimulus without being saturated. Furthermore, given the short duration of the TMS pulse (<1 ms), a preamplifier that limits the rate of voltage change can be utilized to couple TMS with simultaneous EEG without amplifier saturation (24). TMS-EEG can be applied to virtually any cortical area. As a result, concurrent TMS-EEG is a powerful tool to investigate the neuronal properties of neural regions and circuits beyond the motor cortex.
When performing TMS-EEG experiments, 60–200 TMS stimuli should be delivered for each session to obtain a good signal-to-noise ratio and ensure test-retest reliability of EEG responses (25). The intensity of stimulation can be determined as a percentage of the resting motor threshold (% RMT). While %RMT is usually used in TMS-EEG protocols, another approach involves using a TMS neuronavigational system and adjusting in real-time the intensity of stimulation based on the estimated electric field (E-field, expressed as V/m) generated by the TMS in the brain areas of interest. For example, an E-field corresponding to a %RMT can be determined in the motor cortex, and the same intensity can be applied to a non-motor cortical area. A neuronavigational system can also be utilized to more precisely identify and target a given cortical areas which, otherwise, can be indirectly inferred based on the EEG scalp electrodes. For a more detailed description of the TMS-EEG methodology, which exceeds the scope of this review, please refer to (5).
TMS-EEG to examine neural circuits in healthy individuals
TMS-evoked EEG Potentials (TEPs) consist of several peaks and troughs at specific latencies which last several hundred milliseconds after the TMS pulse (Figure 1). In healthy individuals, TEPs can be utilized for several purposes, including: 1) measuring cortical inhibition and excitation; 2) assessing cortical oscillatory activity; and 3) examining cortico-cortical connectivity.
Figure 1. Transcranial Magnetic Stimulation (TMS) as a tool to examine and modulate human brain circuits.
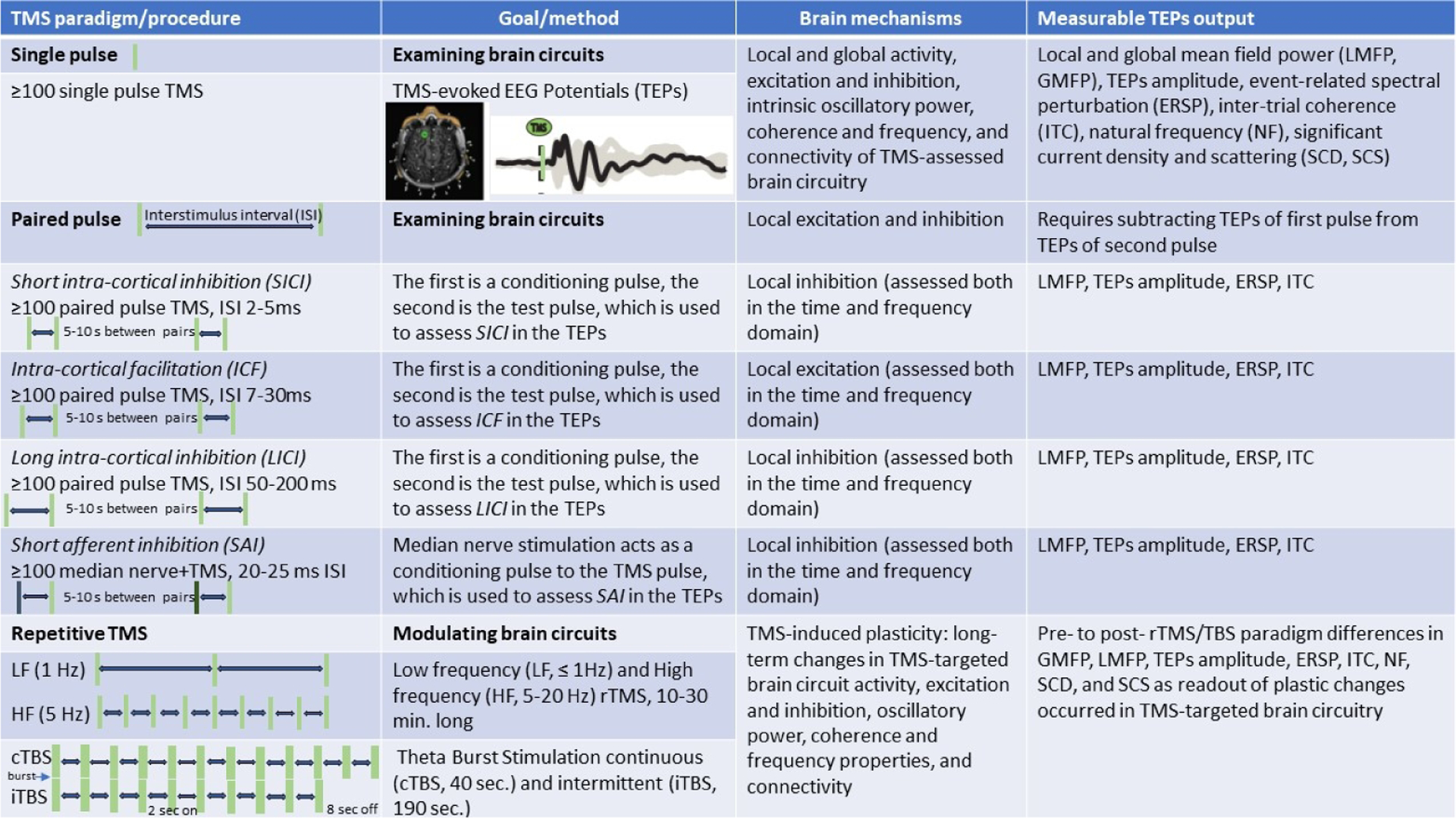
TMS paradigms (single pulse, paired pulse, repetitive TMS) and procedures (number of stimuli, interstimulus interval), goals (probing or modulating) and method (collecting TMS-evoked EEG potentials, or TEPs), brain mechanisms (local and global activity, excitation, inhibition, oscillatory activity, and connectivity), and measurable TEPs output (GMFP, LMFP, TEPs amplitude, ERSP, ITC, NF, SCD, and SCS) are presented.
TMS-assessed cortical excitation, inhibition, and oscillatory properties
The amplitude of TEP peaks and troughs obtained with single pulse TMS protocols can be measured to assess cortical excitation and inhibition. For example, the peak-to-peak amplitude of the N15 and P30 TEP components of the motor cortex was correlated with the amplitude of the MEPs, thus representing a measure of motor cortical excitability (26). On the other hand, TMS-EEG experiments conducted during pharmacological manipulation have shown that N45 and N100 reflect cortical inhibition, representing GABAA and GABAB inhibitory activity, respectively (27). Also, measuring the area under the curve of the rectified TEP signal can provide information about the overall mean field power, which can be calculated locally, local mean field power (LMFP) which is where the TMS is applied, or globally, global field mean power (GMFP). LMFP, which is also called cortical evoked activity (CEA), and GMFP are less affected by variability in the width and amplitude of TEPs in providing a quantification of overall brain circuit activity but ignore the polarity of the signals, which complicates the discrimination between excitatory and inhibitory effects. Furthermore, building on previous single-pulse and paired pulse TMS/MEPs paradigms to investigate motor cortex excitation and inhibition, several TMS-EEG protocols, namely long-interval intracortical inhibition (LICI), short-intracortical inhibition (SICI), intracortical facilitation (ICF), and short-latency afferent inhibition (SAI) have been developed to assess inhibition and excitation in motor and non-motor cortical areas (Figure 1). LICI occurs when two suprathreshold TMS stimuli are applied between 50 and 200 ms, characterized by a reduction in the response to the second stimulus 50–150 ms after TMS, and is thought to reflect GABA-B neuro-transmission (19). A suppression of several TEPs components following LICI has been observed in both motor and prefrontal cortical areas in healthy individuals (27, 28). In SICI, a first, lower intensity stimulus inhibits a second, higher intensity TMS pulse at an interstimulus interval (ISI) of 2–5 ms, whereas longer ISIs (7–30 ms) result in ICF. SICI is associated with GABA-A activity, while IFC relies on both GABA-A and NMDA neurotransmission (29). TMS-EEG studies have shown that early TEPs components are decreased after SICI and increased following ICF in both motor and prefrontal cortical areas (30, 31). A TMS-paradigm of SAI involves the combination of median nerve stimulation with TMS delivered at an ISI of 20–25 ms, and is mainly related to cholinergic and GABAergic activity (29). TMS-EEG studies of SAI have shown a modulation of early components of TEPs in both motor and prefrontal areas in healthy individuals (32). Altogether, findings from these TMS-EEG studies began revealing the neuronal and molecular mechanisms regulating the balance between excitation and inhibition within human cortical areas.
TMS-EEG has also been utilized to assess modulations of cortical excitability and cortical plasticity. Specifically, one study measured the TMS-evoked EEG responses before and after a single dose of levodopa, a compound that is used as a dopamine replacement agent in the treatment of Parkinson Disease (PD), in PD patients. After levodopa intake, an increase in cortical excitability in the supplementary motor areas, but not in the superior parietal lobule, was found that was greater on the more affected side of the brain (33). In another study, wherein TMS-EEG recordings were performed before and after anodal or sham tDCS coupled with a verbal fluency task in healthy individuals, an increase in TMS-evoked EEG responses occurred only after anodal tDCS. This increase was observed in areas involved in language production and it was associated with the degree of cognitive enhancement (34).
TMS-EEG can also be used to assess the oscillatory properties, including power and synchronization, of different cortical areas. Neuronal oscillations are phylogenetically preserved and reflect the physical architecture of neuronal networks, including the number of excitatory and inhibitory interneurons, and their functional characteristics (35). Increasing evidence indicates that neuronal oscillations are critically implicated in human cognition and behavior (36), and that aberrant rhythmic oscillatory activity is commonly observed in psychiatric patients (35, 37). Cortical oscillations tend to occur in specific frequency bands and their activity and level of synchronization may vary across behavioral states and cognitive processes. Thus, while TMS-related measures of cortical excitation and inhibition are mostly collected in the time domain as an increase or decrease in the amplitude of TEPs components, investigations of cortical oscillations are primarily performed in the frequency domain. The event-related spectral perturbation (ERSP) is the parameter most commonly computed to measure the TMS-related activity in a given frequency band whereas the inter-trail coherence (ITC) provides an assessment of the synchronization of the TMS-evoked EEG response across trials (Supp. Fig. 1). Furthermore, by directly probing the cortical surface, TMS-evoked EEG responses can help determine the main oscillatory frequency, or natural frequency (NF), of cortical circuits. TMS-EEG studies from our and other groups have characterized the oscillatory properties of various cortical areas and demonstrated that each of those areas oscillates at a preferred NF, specifically the alpha band (8–12 Hz) for the occipital cortex (38), the low beta range (15–19 Hz) for the parietal cortex (38, 39), and fast beta- and gamma-band for frontal cortical regions, including motor (20–24 Hz) (39), premotor (25–29Hz) (38, 39), and prefrontal (≥30Hz) (39) cortices.
TMS as a probe of cortico-cortical effective connectivity
TMS-EEG also allows the study of neural regional connectivity with enhanced temporal resolution. Neuroimaging techniques, including fMRI and PET, rely on changes in blood flow that can be measured every few seconds whereas TMS-evoked responses can be characterized at the ms scale, the timing of neuronal activity. Furthermore, while neuroimaging signals are based on temporal correlations of vascular (BOLD, rCBF) activities across different neural regions, therefore providing a measure of functional connectivity, TMS-evoked EEG responses can measure effective connectivity, which is the ability of a cortical area to influence the activity of other brain regions thereby assessing the putative directionality and causality of changes in activation. Effective connectivity can be measured through the spatio-temporal dissemination of TEPs and/or TMS-evoked oscillatory activity (40). Source localization refers to the attempt to identify the neuronal sources underlying scalp recorded EEG signals, which is also described as solving the inverse problem of the EEG. Because the possible neuronal sources are far more than the EEG voltages recorded, the inverse problem does not have a unique solution and therefore represents the best approximation based on the available data. Furthermore, neighboring scalp electrodes tend to record similar time series due to volume conduction through non-excitable tissue between depolarizing neurons and recording electrodes, thus creating artificial common sources. Also, the same sensor can record activity from multiple neuronal sources such that two instantaneously interacting (i.e., zero phase lag) sources are difficult to distinguish from a single source whose activity is recorded by the same scalp electrode (41). These challenges can be mitigated by performing high density (N>60 channel) EEG montage, utilizing individual MRI, and employing data analysis tools such as debiased weighted phase lag index (42). Source modeling measures can therefore be used to identify the brain regions underlying scalp-recorded EEG signals, and these measures have been introduced to quantify the distribution of TMS-evoked cortical currents (significant current density, SCD), and the propagation of cortical currents (significant current scattering, SCS) (43).
rTMS and TBS as neuro-modulatory paradigms for neural circuits in healthy individuals
TMS can be employed to modulate the activity of neural circuits. This is usually achieved through repeated stimulation paradigms which include repetitive transcranial magnetic stimulation (rTMS) and theta-burst stimulation (TBS).
rTMS/TBS-induced changes in neural circuits assessed with EEG and behavioral measures
rTMS can induce modifications of synaptic efficacy that outlast the period of stimulation, which in turn determines changes in local cortical excitability. Pharmacological and animal evidence indicates that rTMS affects the neural processes involved in the initiation and maintenance of synaptic plasticity, and especially the long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission (44, 45). The molecular mechanisms associated with TMS-induced changes likely involve NMDA receptors located on the postsynaptic membrane (46, 47). LTP-like effects likely occur through a rapid postsynaptic increase in Ca+ influx followed by increased gene and protein expression whereas a small and slow flow of calcium ions induces LTD-like effects by reducing postsynaptic neuronal activity (47). When applied over the motor cortex, low frequency (LF) rTMS (≤1 Hz) usually decreases whereas high frequency (HF) rTMS (≥5 Hz) increases motor responsiveness, as assessed with MEPs (18), as well as motor performance in both healthy subjects (48) and neuropsychiatric patients (49). The inhibitory effects of low-frequency rTMS have been recently confirmed by a TMS-EEG study in healthy individuals. Specifically, the amplitude of GABA-regulated TMS-evoked early negative EEG components was increased and the amplitude of the MEPs was decreased after 1Hz rTMS of the motor cortex, thus suggesting motor cortical inhibition (50). It is important, however, to take into account that there is a significant across day variability of rTMS effects as shown by a study examining the modulation of corticospinal excitability at various frequencies (1, 10, 20 Hz) and at different time-points in healthy subjects (51). The modulatory effects of rTMS can also be observed in neural regions anatomically connected to the target area, as shown by another TMS-EEG study establishing that 5 Hz rTMS applied to the motor cortex increased the amplitudes of TEPs in bilateral premotor cortices (52). Several animal and imaging studies have also shown that long-distance rTMS effects are mediated through white matter connectivity and that rTMS can be utilized to modulate local oscillations and interregional synchrony, as reported in a recent elegant review (53).
TBS is a repetitive TMS protocol that employs short bursts (three pulses at 50 Hz) delivered at a frequency of 5 Hz that can both increase and decrease the excitability of cortical neurons (54). Such theta burst leads to a short-latency facilitation, likely related to the (fast) rate of postsynaptic calcium inflow, with a longer-latency and weaker inhibition due to the overall amount of calcium entry (55). TBS can be applied intermittently (intermittent TBS, or iTBS), which consists of twenty 2 s trains interleaved with periods of silence (~8 s), for a total duration of ~190 s, or continuously (continuous TBS, or cTBS), for a total of 40 s, both of which are significantly shorter than rTMS paradigms, thus allowing for TBS to induce more rapid effects on neural activity than conventional rTMS (56). When applied to the motor cortex, iTBS leads to an increase whereas cTBS determines a decrease in corticospinal excitability as reflected in changes in MEP amplitude (57). Furthermore, a reduction in the TMS-evoked EEG responses, assessed both in the time domain (LMFP) and frequency domain (ERSP, ITC), has been observed in the theta frequency range following cTBS of the motor cortex (58). In contrast, iTBS of the dorsolateral prefrontal cortex (DLPFC) increased both the amplitude of early TEPs (i.e., N120) and the power of TMS-evoked theta oscillations, assessed with single pulse TMS, as well as the LICI of theta oscillations, evaluated with paired-pulse TMS, in healthy individuals (59). Two recent studies showed that cTBS to somatosensory cortex interfered with normal sensory function, and that it blocked motor memory consolidation, but not the ability to retrieve a consolidated motor memory (60), and that cTBS of the visual cortex, but not of a control region (vertex), applied immediately after the offset of a visual task training interfered with the consolidation of visual perceptual learning (61). Together, these findings suggest that this approach can elucidate understanding of neural circuit-behavior relationships. It is important, however, to point out that the excitatory effects of HF rTMS and iTBS and the inhibitory effects of LF rTMS and cTBS have been observed primarily in the motor cortex with group level analyses, and therefore their ultimate effects on individual subjects and non-motor cortical areas still need to be fully established. Furthermore, a limitation of this approach is that the TMS frequency refers to the repetition rate of a given stimulation pulse that in turn has a specific pulse width; thus, TMS does not represent an ideal method to entrain a given oscillation.
TMS as an examination and a neuromodulatory tool in psychiatry
TMS has been utilized to establish dysfunctions of the excitatory and inhibitory properties, the oscillatory activity, and the connectivity of cortical areas in individuals with psychiatric disorders (Box 1). As a result of their ability to induce changes in the activity and connectivity of neural circuits that outlast the duration of stimulation, rTMS and, more recently, TBS, have been increasingly utilized in psychiatric populations as a potential treatment (11). Furthermore, since electroconvulsive therapy (ECT) is arguably the most effective intervention for several treatment-resistant psychiatric disorders, including schizophrenia and mood disorders, NIBS techniques such as rTMS and TBS have emerged as promising treatment options that require less intense stimulation than ECT while also providing more focal interventions and increased specificity of the neural targets being stimulated although perspective, properly randomized clinical trials comparing the efficacy of these interventions are needed in psychiatric patients (Box 1). Here, we focus on TMS findings in schizophrenia and mood disorder patients, the psychiatric disorders most studied using this technique
Box 1. TMS: present practices in psychiatry.
TMS as a probe
TMS-assessed EEG abnormalities of cortical excitation (ICF), inhibition (SICI, LICI, SAI), and oscillatory activity (ERSP, ITC, NF) of different cortical areas (parietal, motor, premotor, prefrontal) in psychiatric patients
TMS-related EEG measures of altered cortico-cortical connectivity (SCS, SCD) in psychiatric populations
TMS as a treatment tool
rTMS (LF, HF) and TBS (cTBS, iTBS), sham-controlled paradigms applied in different combinations (ipsilateral, bilateral simultaneous, bilateral sequential, priming) to ameliorate clinical symptoms in psychiatric patients.
Symptom improvement is the target and the main outcome measure, although baseline rs-fMRI functional connectivity patterns are being increasingly used to both guide treatment and assess treatment response
TMS findings in schizophrenia
Schizophrenia is a psychiatric disorder characterized by positive (i.e., hallucinations, delusions), negative (i.e., emotional and social withdrawal), and cognitive symptoms with a lifetime prevalence of ~1% (62). Although many brain regions and molecular mechanisms are implicated in the pathophysiology of this disorder, including abnormalities in the mesocortical dopaminergic pathway (63) as well as in hippocampal (64) and thalamocortical(64) glutamatergic activity, converging post-mortem and electrophysiological evidence point to alterations in frontal-prefrontal cortical areas and in GABA-ergic neurotransmission in schizophrenia (65, 66). Specifically, molecular abnormalities have been consistently reported in GABAergic cortical inhibitory neurons by several human post-mortem studies in patients with schizophrenia (67). Furthermore, aberrant fast, beta and gamma oscillations, which are thought to be generated and modulated by GABA-ergic neurotransmission(68), have been reported in numerous studies in schizophrenia patients (37). Building on these findings, TMS-EEG has been increasingly employed to directly assess the intrinsic properties of local cortical neurons as well as cortico-cortical connectivity, while rTMS and TBS paradigms have been utilized to modulated these cortical properties in schizophrenia patients, as detailed below.
TMS-assessed abnormalities of cortical excitation, inhibition, and oscillatory activity in schizophrenia
Concerning TMS/MEP findings, RMT and MEP amplitude are the most reported measures of motor cortical excitability. In a recent meta-analysis of twenty-one studies, RMT did not differ between patients with schizophrenia (N = 500) and healthy subjects (N = 617) across 21 studies(69). Similarly, MEP amplitude did not differ between patients with schizophrenia and healthy controls across eight studies (7). Furthermore, no differences were found in ICF in individuals at risk of schizophrenia, first-episode, and both medicated and unmedicated chronic SCZ relative to healthy control groups (7, 19). TMS/MEP studies have also investigated motor cortical inhibition, and reduced SICI has been reported in patients at various stages of illness, including individuals at risk of developing schizophrenia, first-episode patients, recent onset patients, and chronic patients (19).
Several recent TMS-EEG studies have characterized these alterations in individuals with schizophrenia. For example, in an initial TMS-EEG study, we showed a reduction in the GMFP of the early TEPs as well as in TMS-assessed oscillatory properties, including amplitude, assessed with ERSP, and synchronization, assessed with ITC, of the premotor cortex, in individuals with schizophrenia relative to healthy individuals (70). In a follow-up study, where we investigated the oscillatory properties of four cortical areas, parietal, motor, premotor, and prefrontal cortices, individuals with schizophrenia showed a reduction in TMS-related amplitude (ERSP) and synchronization (ITC) of beta/gamma oscillations in frontal/prefrontal, but not in parietal, regions compared with healthy individuals (39). Furthermore, individuals with schizophrenia showed a slowing in the natural frequency of frontal/prefrontal regions compared with healthy individuals, from a mean 2-Hz decrease for the motor area to an almost 10-Hz decrease for the prefrontal cortex, to the extent that the prefrontal natural frequency of individuals with schizophrenia was slower than that of any healthy individual. These findings point to intrinsic abnormalities in frontal/prefrontal cortical neurons, in schizophrenia, which are likely mediated by alterations in GABA-ergic activity. Specifically, modeling and in vivo electrophysiological animal studies have demonstrated that inhibiting fast-spiking GABA-ergic interneurons suppressed power and synchronization of gamma oscillations whereas driving these interneurons was sufficient to generate γ-band rhythmicity (71, 72). Abnormalities in prefrontal cortical inhibition have been reported by other TMS-EEG studies, which have shown a reduced inhibition of gamma oscillations induced by LICI in the dorsolateral prefrontal cortex, but not in the motor cortex, of individuals with schizophrenia and their first-degree relatives compared with healthy individuals (73).
TMS as a probe of altered effective connectivity in schizophrenia
TMS-evoked EEG responses have also been utilized to study alterations in cortico-cortical connectivity in schizophrenia. One TMS-EEG study examining the motor cortex showed an aberrant, widespread pattern of propagation in individuals with schizophrenia, which was observed for several hundred ms after the TMS pulse (i.e., between 400 and 700 ms post TMS) and was characterized by an increase in global TMS-evoked voltage activity and enhanced fast oscillations in fronto-parietal regions, relative to healthy individuals (74). By targeting the premotor cortex in both schizophrenia patients and control subjects, and by performing source modeling analysis of the TMS-evoked EEG responses, we previously demonstrated that, in healthy individuals, TMS-evoked cortical activity propagated from the premotor to other functional and anatomically connected cortical areas (right sensorimotor areas and left premotor and sensorimotor regions), whereas in individuals with schizophrenia, the evoked activity was mostly localized to the stimulated area (70). Furthermore, by utilizing source-based measures of TMS-evoked cortical activity (SCD) and connectivity (SCS), there were reductions in SCD and SCS in premotor/prefrontal areas which were associated with impaired cognitive function in individuals with schizophrenia relative to healthy individuals (75).
rTMS/TBS as interventions in schizophrenia
LF, 1 Hz rTMS of left temporo-parietal cortex has been the most consistently used paradigm to treat auditory hallucinations in schizophrenia. Auditory hallucinations are a core symptom of schizophrenia that is thought to emerge from the hyperactivation in temporo-parietal regions involved in auditory and speech processing, as shown by electrophysiological and neuroimaging studies (76). A recent meta-analysis comparing active vs sham stimulation reported a medium effect size (ES= 0.51), and found that younger age, female gender, higher antipsychotic doses, brief (<3 week) trial duration, and shorter scalp-to-temporal cortex distance predicted a better response to treatment (77). The improvement of auditory hallucinations was relatively short-lasting (4–6 weeks), however, and did not extend to other psychotic symptoms. Furthermore, other rTMS protocols, including cTBS, have failed to show a consistent improvement of auditory hallucination or related psychotic symptoms (15).
Negative symptoms are a core feature of schizophrenia that is often resistant to treatment with antipsychotic compounds. Evidence regarding the efficacy of rTMS on negative symptoms is mixed. Two meta-analyses showed that active rTMS (≥5 Hz) was more effective than sham rTMS, corresponding to small-to-medium effect sizes (ES=0.49–0.64) (77, 78), whereas a third metanalysis (79) found no differences between these two interventions. Furthermore, in a recent randomized clinical trial wherein schizophrenia patients received 20 sessions of active or sham rTMS of left prefrontal cortex at 20 Hz over 4 weeks the improvement in the negative symptoms (Anhedonia, Alogia, Avolition, Attention impairment) in the active group was statistically significant as compared to the sham group (80). Of note, most of these studies targeted the prefrontal cortex, given that several neuroimaging studies have shown hypometabolism and hypoperfusion of pre-frontal regions in patients suffering from negative symptoms (81). One promising alternative approach, based on the observation that the functional connectivity of the cerebellum with the right prefrontal cortex was inversely associated with negative symptom severity, has been to impact prefrontal activity by modulating the cerebellum. In a recent study, the iTBS of the cerebellum resulted in improvement of negative symptoms and reversal of cerebellar-prefrontal functional dysconnectivity in patients with schizophrenia(82).
Cognitive deficits represent one of the most persistent, treatment-resistant features of schizophrenia. Most neuromodulation studies employed HF (10–20 Hz) rTMS to the left or bilateral DLPFC, given the critical role of the cortical area in the cognitive dysfunctions of schizophrenia(83). Pilot data from a randomized clinical trial showed that 20-Hz active rTMS over 4 weeks was associated with significant improvement in working memory compared with sham rTMS in individuals with schizophrenia (84). Another pilot study found that individuals with schizophrenia receiving bilateral 20-Hz active rTMS for 2 weeks, compared with sham stimulation, showed an improvement on a standardized cognitive battery both immediately following treatment and two weeks later (85). In contrast, a large, multi-center study found no benefits of 3-week left prefrontal 10-Hz stimulation, when compared with sham stimulation, in individuals with schizophrenia (86). Furthermore, a recent meta-analysis of cognitive benefits with rTMS in schizophrenia showed significant efficacy of high frequency rTMS on working memory when compared with sham stimulation, which corresponded to an ES=0.34 and persisted at a 1-month follow-up assessment (87). Overall, despite some positive findings, the relatively small degree of improvement restricted to specific cognitive domains displays the need to develop novel neuromodulation protocols, including TBS paradigms, to effectively enhance cognitive function in individuals with schizophrenia.
TMS findings in Bipolar Disorder (BD) and Major Depressive Disorder (MDD)
BD is a group of brain disorders characterized by a history of hypo/manic episodes, periods of elevated/irritable mood and energized behaviors (88). With a lifetime prevalence of more than 2%, BD represents one of the leading causes of disability worldwide (89). In a critical review of neuroimaging findings, we recently reported that functional MRI studies suggest a dysfunction in a ventrolateral prefrontal-hippocampal-amygdala circuit bilaterally combined with an hyperactive left-sided ventral striatal-ventrolateral and orbitofrontal cortical reward-processing circuits, which would lead to emotion dysregulation and heightened reward sensitivity respectively (90). Furthermore, we found that structural imaging findings point gray matter volume decreases in the prefrontal and temporal cortices, the amygdala, and the hippocampus as well as fractional anisotropy decreases in white matter tracts connecting prefrontal and subcortical regions (90).
MDD is the most common mood disorder, and it is characterized by depressed mood or loss of interest and pleasure in daily activities along with several vegetative and psychological symptoms (88). Regarding the pathophysiology of MDD, the traditional monoamine hypothesis postulates that depression is caused by disrupted dopaminergic, noradrenergic, and serotonergic neurotransmission as suggested by the antidepressant effects of monoaminergic agents (91). Although this hypothesis remains highly influential, more recent theories have focused on altered neuronal interaction and disturbed Glutamatergic and GABAergic neurotransmission as critically implicated in the neurobiology of depression. For example, the neuroplasticity theory of depression hypothesizes that impaired neuroplasticity is the cellular basis of depressed mood and contributes to the cognitive bias and impairments which are often present in depressed patients (92), while the “synaptogenic hypothesis of depression” postulates that dysfunctional synaptic transmission, involving primarily Glutamatergic and GABAergic neurotransmission, is a fundamental element of the pathophysiology of MDD (93). Clinical studies have shown altered glutamate levels in the serum, cerebrospinal fluid and central nervous system of depressed patients, along with altered glutamatergic NMDA receptor activity in postmortem analyses (94), whereas altered GABA concentrations have been reported in the prefrontal cortex of depressed patients using MR spectroscopy (95) and in postmortem studies showing a reduction of cortical GABAergic neurons (96).
Because of its ability to serve both as an examining and a neuro-modulatory tool, TMS can be employed to characterize and possibly ameliorate these neuronal and molecular dysfunctions in BD and MDD patients, as outlined below.
TMS-assessed abnormalities of cortical excitation, inhibition, and oscillatory activity in mood disorders
Concerning TMS/MEP findings, in a meta-analysis of excitability measures in patients with MDD and healthy controls, no group differences were found in RMT (MDD: N = 176; healthy controls: N = 188) or ICF (MDD: N = 115; healthy controls: N = 130) (69). Three of these studies also measured MEP amplitude, and reported no differences between MDD and healthy controls(69). In the only TMS-EEG study that involved both individuals with BD and those with MDD, wherein the main natural oscillatory frequency of a frontal area, the premotor cortex, was the main outcome measure, both patient populations, along with a group of patients with SCZ, showed a reduction in the premotor natural frequency relative to healthy individuals (97). Another study found that individuals with MDD had a higher cortical reactivity, assessed with the GMFP, and a stronger inhibitory response, reflected by larger negative peaks, in the DLPFC compared with healthy individuals. Furthermore, these TMS-evoked EEG measures were positively correlated with each other in healthy individuals, but not in individuals with MDD, suggesting an imbalance between excitation and inhibition in MDD (98). It is important, however, to point out that this study did not account for the TMS click auditory response nor employed a sham condition; thus, its main findings should be replicated in future work employing these TMS control conditions.
TMS as a probe of altered effective connectivity in mood disorders
An increase in DLPFC cortical reactivity, but not in motor or parietal cortical areas, along with increased cortico-cortical connectivity was also recently reported in youth with MDD relative to healthy youth, and the increase in cortical reactivity correlated with anhedonia severity in the former (99).
rTMS/TBS as interventions in mood disorders
Among psychiatric disorders, rTMS and TBS paradigms have shown the strongest evidence of the efficacy in MDD. The Food and Drug Administration (FDA) approved rTMS in 2008 and TBS in 2018 for the treatment of MDD. In addition, according to the Canadian Network for Mood and Anxiety Treatments (CANMAT), rTMS is a first-line treatment for individuals which have failed at least one antidepressant trial. Major depressive disorders are characterized by metabolic and neuronal activity asymmetry in the two prefrontal areas, which consists of enhanced glucose and oxygen consumption along with higher EEG activity on the right side, combined with reduced activation on the left side (100). Thus, the most employed stimulation paradigms in MDD have been HF (≥10 Hz) rTMS targeted to the left DLPFC and LF (≤1 Hz) rTMS targeted to the right DLPFC. Other TMS treatment paradigms involved the combination of HF and LF protocols, which can be administered as “bilateral” rTMS, with HF and LF being applied simultaneously or, more commonly, sequentially, to contralateral cortical areas and “priming” rTMS, when a rTMS protocol is applied to the same neural region to boost the effects of a second paradigm (e.g., HF before LF to augment LF effects) (11).
A recent network meta-analysis evaluated the efficacy and tolerability of different TMS protocols with each active treatment compared with sham stimulation (101). Treatments that were more effective than sham included HF-, LF-, bilateral, priming rTMS and TBS. A meta-analysis specifically assessing the clinical efficacy and safety of rTMS in BD depression reported better responses to LF-rTMS over the right DLPFC than HF-rTMS over the left DLPFC when compared with sham stimulation although the studies showed a considerable methodological heterogeneity and included relatively small sample sizes (102). Randomized clinical trials have shown that rTMS paradigms have comparable efficacy to antidepressant treatments, including individuals with moderate to high degrees of refractoriness (103). Together, these findings indicate that both rTMS and TBS are effective treatment interventions for MDD, whereas more evidence is needed to demonstrate their efficacy in individuals with treatment resistant MDD or BD.
Challenges and open questions
To reach its full potential, the combination of TMS-EEG with rTMS/TBS will require addressing the challenges and current limitations of each of these techniques. Regarding TMS-EEG, one challenge is the influence of medications on TMS-evoked activity, given that most individuals with psychiatric disorders are medicated at the time of the assessment. In addition to recruiting unmedicated patients, a way to address this issue involves testing participants in the early stages of illness, when they are still medication naïve or minimally treated. In recent work, we have shown the feasibility of this approach in first-episode psychosis individuals (104). Other challenges include ensuring that the TMS coil is properly placed and maintained over the cortical area of interest throughout the TMS-EEG session. The use of TMS neuronavigational devices has shown to be effective in minimizing this possible confound, however. Another common pitfall associated with the TMS-EEG procedure include somatosensory evoked potentials related to superficial scalp activation from the TMS pulse as well as auditory evoked potentials to the TMS discharge sound that can contaminate the TMS-evoked EEG responses to direct cortical activation. Previous work from our and other research groups has shown that these potential contaminants can be substantially mitigated through the placement of a foam layer underneath the coil and with auditory noise masking (38, 39). Nonetheless, concerns remain about the challenge of disentangling genuine cortical responses to TMS from those resulting from concomitant sensory activation (105), which warrants the development of standard procedures in TMS-EEG studies (106, 107). Similarly, although TMS-EEG data analyses have developed significantly since the technique was first introduced, and some open-source TMS-EEG analysis software are currently available, there is still relatively large inter-individuals variability of the TMS-related measures (108, 109) which shows a need to develop standardized pre-processing and post-processing analysis pipelines (16) and has brought about some important initiatives, such as the Big TMS data collaboration. Regarding the rTMS/TBS paradigms, in addition to identifying the ideal cortical area to maximize treatment response, which might require combining clinical, neuroimaging, and neurophysiological information, it will be important to optimize the patterns and frequencies of stimulation. This is especially relevant for TBS, which can induce opposite effects on the motor cortex by modifying the pattern, but not the frequency, of stimulation. Future work should, therefore, establish these effects in non-motor areas and develop the TBS paradigms that best modulate the activity of these areas in the desired direction. Other factors to consider are the TMS dose to apply, given that an accelerated, high-dose, iTBS protocol delivered in an unblinded study was found to be safe and induced remission in 19 of 22 individuals with TRD (110), controlling the cognitive state (e.g., level of vigilance, rest vs. performing a task while inducing symptoms) of individuals at the same of the rTMS/TBS procedure, and exploring the combination of neuromodulation with other treatment interventions including pharmacotherapy and psychotherapy. Nonetheless, evidence accumulated so far indicates that TMS is uniquely suited to both examine and modulate neural circuit function. Thus, there is huge potential to develop a TMS-based personalized, precision medicine approach to assess, as well as treat, individuals with psychiatric disorders, as outlined in the next and final section.
Conclusions and future directions
Building on this growing body of evidence, we suggest three areas of study for future TMS work in psychiatric disorders. These studies can ultimately contribute to the development of better, neuromodulatory-based interventions for individuals with these disorders: 1) using TMS-EEG to characterize the local and long-range cortical abnormalities that are to be used as neural targets in TMS target engagement studies 2) acutely modulating neural circuits with rTMS/TBS paradigms to determine the impact of such neuromodulation on related biological and clinical parameters, to better inform subsequent neuromodulation-based treatment interventions, and 3) combining neuroimaging, neurophysiological, and clinical measures related to TMS-targeted neural circuits to better predict and track clinical outcomes in TMS clinical trial studies (Box 2).
Box 2. TMS: future developments in psychiatry.
Employing TMS-related EEG measures to elucidate neural circuit dysfunctions, to provide more accurate neural targets for TMS-based interventions in psychiatric disorders.
Acutely modulating neural circuitries with rTMS/TBS paradigms and examining their impact on related biological and clinical parameters, to better inform subsequent neuromodulation-based treatment interventions.
Combining neuroimaging, neurophysiological, and clinical measures related to TMS-targeted neural circuitries to better predict and track clinical outcomes in TMS clinical trial studies.
Addressing challenges and limitations of each of the current approaches (TMS-EEG, rTMS, TBS).
Employing TMS-related EEG measures to elucidate neural circuit dysfunctions, to provide more accurate neural targets for TMS-based interventions in psychiatric disorders.
While TMS-EEG has been utilized to characterize the neurophysiological abnormalities of major psychiatric disorders, TMS-EEG could also be employed in combination with rTMS/TBS paradigms to identify predictive, prognostic, and pathophysiological biomarkers of NIBS-based treatment interventions. For example, a recent study showed that TMS-assessed cortical inhibition of DLPFC predicted the response to a course of Magnetic Seizure Therapy (MST), a rTMS paradigm recently developed as an alternative to ECT in treatment-resistant MDD (TRD) (111). Specifically, a greater decrease in the suicidal ideations was associated with larger pre-MST DLPFC LICI values, suggesting that higher cortical inhibition at baseline is an indicator of remission of suicide ideations (i.e., a predictive biomarker). Another TMS-EEG study in TRD showed an increase in TEP immediate power and slope after several ECT sessions, and that this increase was associated with reduced depression severity (112). Although these findings need to be replicated in larger samples, as well as including rTMS/TBS paradigms, some of these TMS-evoked EEG measures are potential predictive biomarkers of neuromodulation interventions in mood disorders. Furthermore, TMS-EEG neurophysiological measures that are altered in schizophrenia (e.g., LICI (73), generation and modulation of gamma oscillatory activity (16) and natural frequency (39) of the DLPFC) could be utilized to develop rTMS/TBS protocols targeting the DLPFC and/or other interconnected neural regions to delay, halt or even reverse pathophysiological processes and related clinical and functional impairments in individuals with schizophrenia, thereby serving as target engagement biomarkers for NIBS-based treatment interventions.
Acutely modulating neural circuits with rTMS/TBS paradigms and examining their impact on related biological and clinical parameters, to better inform subsequent neuromodulation-based treatment interventions
Increasing evidence indicates that rTMS/TBS paradigms can acutely modulate the activity of neural circuits and related behavioral parameters in healthy individuals as reported above. In contrast, little is known about the acute effects of these paradigms in psychiatric disorders. Traditionally, TMS-based interventions target a given cortical area (i.e., DLPFC), and measure the impact of these interventions on clinical parameters, such as improvement in depression. More recently, functional neuroimaging, and especially those including measures of functional connectivity, have been used to prospectively identify TMS targets for future treatment interventions. One fMRI study of individuals with MDD showed that, compared with responders, non-responders had higher anhedonia and lower connectivity in a neural circuit classically associated with reward, comprising the ventral tegmental area, striatum, and part of the ventromedial prefrontal cortex (113). Another fMRI study, conducted in a large multisite sample, showed that individuals with MDD can be subdivided into four neurophysiological subtypes (‘biotypes’) defined by distinct patterns of dysfunctional connectivity in limbic and fronto-striatal networks (114). These biotypes were associated with differing symptom profiles and predicted responsiveness to TMS therapy. Specifically, individuals in biotype 1 were approximately three times more likely to benefit from rTMS over the dorsomedial prefrontal cortex (DMPFC) than those in biotypes 2 or 4. These biotypes have been hard to replicate, however (115). One of the challenges of relying on these functional biotypes is establishing a causal relationship between brain activity and symptomatology. By acutely modulating with rTMS/TBS paradigms altered neural circuits and examining their immediate impact on related biological and clinical parameters, this challenge can begin to be addressed. Specifically, neuroimaging and/or neurophysiological assessments along with clinical evaluations before and after acute rTMS/TBS protocols can elucidate the causal role of a given neural circuit in the development of specific symptoms or behaviors.
Combining neuroimaging, neurophysiological, and clinical measures related to TMS-targeted neural circuits to better predict and track clinical outcomes in TMS clinical trial studies.
Combining TMS-related EEG parameters with rTMS/TBS paradigms has potential to significantly improve clinical outcomes in psychiatric disorders for the following reasons. First, TEPs can characterize the neurophysiological properties of the cortical area to be targeted by rTMS, which in turn may help monitor treatment response. Consistent with this approach, a randomized, sham-controlled clinical trial of rTMS in MDD combining fMRI and TMS-EEG reported that baseline DLPFC fMRI global connectivity predicted clinical outcome whereas local and distributed changes in TMS-EEG potentials tracked clinical outcome (116). Second, TMS-evoked EEG responses provide information about the effective connectivity of the TMS-targeted cortical area with the whole brain. This information, combined with the functional connectivity maps obtained by fMRI, can better characterize the impact of rTMS on the target neural region and the neural circuit of interest, which, in turn, should lead to a better prediction and monitoring of response to treatment in individuals with psychiatric disorders.
Supplementary Material
Acknowledgments:
This work was supported by the National Institute of Mental Health BRAINS award R01MH113827(FF), R21MH119543 (FF),R37MH100041 (MPL), and the Pittsburgh Foundation (MLP). We would like to thank all patients who agreed to participate to the studies cited in the manuscript.
Footnotes
Disclosure: None.
The authors report no financial relationships with commercial interests.
References
- 1.Boes AD, Kelly MS, Trapp NT, Stern AP, Press DZ, Pascual-Leone A. Noninvasive Brain Stimulation: Challenges and Opportunities for a New Clinical Specialty. J Neuropsychiatry Clin Neurosci. 2018;30:173–179. [DOI] [PubMed] [Google Scholar]
- 2.Gomes-Osman J, Indahlastari A, Fried PJ, Cabral DLF, Rice J, Nissim NR, Aksu S, McLaren ME, Woods AJ. Non-invasive Brain Stimulation: Probing Intracortical Circuits and Improving Cognition in the Aging Brain. Front Aging Neurosci. 2018;10:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh H, Kim JH, Yau JM. EPI distortion correction for concurrent human brain stimulation and imaging at 3T. J Neurosci Methods. 2019;327:108400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobos Sanchez C, Cabello MR, Olozabal AQ, Pantoja MF. Design of TMS coils with reduced Lorentz forces: application to concurrent TMS-fMRI. J Neural Eng. 2020;17:016056. [DOI] [PubMed] [Google Scholar]
- 5.Farzan F, Vernet M, Shafi MM, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and Modulating Brain Circuitry through Transcranial Magnetic Stimulation Combined with Electroencephalography. Front Neural Circuits. 2016;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev. 2016;64:175–184. [DOI] [PubMed] [Google Scholar]
- 7.Kaskie RE, Ferrarelli F. Investigating the neurobiology of schizophrenia and other major psychiatric disorders with Transcranial Magnetic Stimulation. Schizophr Res. 2018;192:30–38. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. [DOI] [PubMed] [Google Scholar]
- 9.Burke MJ, Fried PJ, Pascual-Leone A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. Handb Clin Neurol. 2019;163:73–92. [DOI] [PubMed] [Google Scholar]
- 10.George MS. Whither TMS: A One-Trick Pony or the Beginning of a Neuroscientific Revolution? Am J Psychiatry. 2019;176:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunoni AR, Sampaio-Junior B, Moffa AH, Aparicio LV, Gordon P, Klein I, Rios RM, Razza LB, Loo C, Padberg F, Valiengo L. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry. 2019;41:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rego SR, Marcolin MA, May G, Gjini K. Effects of transcranial magnetic stimulation on the cognitive event-related potential p300: a literature review. Clin EEG Neurosci. 2012;43:285–290. [DOI] [PubMed] [Google Scholar]
- 14.Bestmann S, Feredoes E. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Ann N Y Acad Sci. 2013;1296:11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta UM, Naik SS, Thanki MV, Thirthalli J. Investigational and Therapeutic Applications of Transcranial Magnetic Stimulation in Schizophrenia. Curr Psychiatry Rep. 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, Di Lazzaro V, Farzan F, Ferrarelli F, Fitzgerald PB, Hui J, Ilmoniemi RJ, Kimiskidis VK, Kugiumtzis D, Lioumis P, Pascual-Leone A, Pellicciari MC, Rajji T, Thut G, Zomorrodi R, Ziemann U, Daskalakis ZJ. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130:802–844. [DOI] [PubMed] [Google Scholar]
- 17.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. [DOI] [PubMed] [Google Scholar]
- 18.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr Bull. 2014;40:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. [DOI] [PubMed] [Google Scholar]
- 21.Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–424. [DOI] [PubMed] [Google Scholar]
- 22.Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. [DOI] [PubMed] [Google Scholar]
- 23.Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput. 1999;37:322–326. [DOI] [PubMed] [Google Scholar]
- 24.Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clin Neurophysiol. 2006;117:1870–1875. [DOI] [PubMed] [Google Scholar]
- 25.Kerwin LJ, Keller CJ, Wu W, Narayan M, Etkin A. Test-retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimul. 2018;11:536–544. [DOI] [PubMed] [Google Scholar]
- 26.Maki H, Ilmoniemi RJ. The relationship between peripheral and early cortical activation induced by transcranial magnetic stimulation. Neurosci Lett. 2010;478:24–28. [DOI] [PubMed] [Google Scholar]
- 27.Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U, Muller-Dahlhaus F. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. Neuroimage. 2014;103:152–162. [DOI] [PubMed] [Google Scholar]
- 28.Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition of distinct mechanisms in the dorsolateral prefrontal cortex is related to working memory performance: a TMS-EEG study. Cortex. 2015;64:68–77. [DOI] [PubMed] [Google Scholar]
- 29.Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Muller-Dahlhaus F. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. [DOI] [PubMed] [Google Scholar]
- 30.Ferreri F, Pasqualetti P, Maatta S, Ponzo D, Ferrarelli F, Tononi G, Mervaala E, Miniussi C, Rossini PM. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2011;54:90–102. [DOI] [PubMed] [Google Scholar]
- 31.Cash RF, Noda Y, Zomorrodi R, Radhu N, Farzan F, Rajji TK, Fitzgerald PB, Chen R, Daskalakis ZJ, Blumberger DM. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG. Neuropsychopharmacology. 2017;42:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noda Y, Cash RF, Zomorrodi R, Dominguez LG, Farzan F, Rajji TK, Barr MS, Chen R, Daskalakis ZJ, Blumberger DM. A combined TMS-EEG study of short-latency afferent inhibition in the motor and dorsolateral prefrontal cortex. J Neurophysiol. 2016;116:938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casarotto S, Turco F, Comanducci A, Perretti A, Marotta G, Pezzoli G, Rosanova M, Isaias IU. Excitability of the supplementary motor area in Parkinson’s disease depends on subcortical damage. Brain Stimul. 2019;12:152–160. [DOI] [PubMed] [Google Scholar]
- 34.Pisoni A, Mattavelli G, Papagno C, Rosanova M, Casali AG, Romero Lauro LJ. Cognitive Enhancement Induced by Anodal tDCS Drives Circuit-Specific Cortical Plasticity. Cereb Cortex. 2018;28:1132–1140. [DOI] [PubMed] [Google Scholar]
- 35.Buzsaki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14:345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Experiments Frohlich F. and models of cortical oscillations as a target for noninvasive brain stimulation. Prog Brain Res. 2015;222:41–73. [DOI] [PubMed] [Google Scholar]
- 37.Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 2015;77:1001–1009. [DOI] [PubMed] [Google Scholar]
- 38.Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, Massimini M, Postle BR, Tononi G. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry. 2012;69:766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bortoletto M, Veniero D, Thut G, Miniussi C. The contribution of TMS-EEG coregistration in the exploration of the human cortical connectome. Neurosci Biobehav Rev. 2015;49:114–124. [DOI] [PubMed] [Google Scholar]
- 41.Palva JM, Wang SH, Palva S, Zhigalov A, Monto S, Brookes MJ, Schoffelen JM, Jerbi K. Ghost interactions in MEG/EEG source space: A note of caution on inter-areal coupling measures. Neuroimage. 2018;173:632–643. [DOI] [PubMed] [Google Scholar]
- 42.Alagapan S, Riddle J, Huang WA, Hadar E, Shin HW, Frohlich F. Network-Targeted, Multi-site Direct Cortical Stimulation Enhances Working Memory by Modulating Phase Lag of Low-Frequency Oscillations. Cell Rep. 2019;29:2590–2598 e2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M. General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage. 2010;49:1459–1468. [DOI] [PubMed] [Google Scholar]
- 44.Lefaucheur JP. Neurophysiology of cortical stimulation. Int Rev Neurobiol. 2012;107:57–85. [DOI] [PubMed] [Google Scholar]
- 45.Cheeran B, Koch G, Stagg CJ, Baig F, Teo J. Transcranial magnetic stimulation: from neurophysiology to pharmacology, molecular biology and genomics. Neuroscientist. 2010;16:210–221. [DOI] [PubMed] [Google Scholar]
- 46.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. [DOI] [PubMed] [Google Scholar]
- 47.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front Hum Neurosci. 2015;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park CH, Chang WH, Yoo WK, Shin YI, Kim ST, Kim YH. Brain topological correlates of motor performance changes after repetitive transcranial magnetic stimulation. Brain Connect. 2014;4:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson’s disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44:892–898. [DOI] [PubMed] [Google Scholar]
- 50.Casula EP, Tarantino V, Basso D, Arcara G, Marino G, Toffolo GM, Rothwell JC, Bisiacchi PS. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. Neuroimage. 2014;98:225–232. [DOI] [PubMed] [Google Scholar]
- 51.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. [DOI] [PubMed] [Google Scholar]
- 52.Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. [DOI] [PubMed] [Google Scholar]
- 53.Valero-Cabre A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381–404. [DOI] [PubMed] [Google Scholar]
- 54.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. [DOI] [PubMed] [Google Scholar]
- 55.Huang YZ, Rothwell JC, Chen RS, Lu CS, Chuang WL. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Downar J. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–1692. [DOI] [PubMed] [Google Scholar]
- 57.Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul. 2016;9:323–335. [DOI] [PubMed] [Google Scholar]
- 58.Rocchi L, Ibanez J, Benussi A, Hannah R, Rawji V, Casula E, Rothwell J. Variability and Predictors of Response to Continuous Theta Burst Stimulation: A TMS-EEG Study. Front Neurosci. 2018;12:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung SW, Lewis BP, Rogasch NC, Saeki T, Thomson RH, Hoy KE, Bailey NW, Fitzgerald PB. Demonstration of short-term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: A TMS-EEG study. Clin Neurophysiol. 2017;128:1117–1126. [DOI] [PubMed] [Google Scholar]
- 60.Kumar N, Manning TF, Ostry DJ. Somatosensory cortex participates in the consolidation of human motor memory. PLoS Biol. 2019;17:e3000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bang JW, Milton D, Sasaki Y, Watanabe T, Rahnev D. Post-training TMS abolishes performance improvement and releases future learning from interference. Commun Biol. 2019;2:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreno-Kustner B, Martin C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One. 2018;13:e0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019;42:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallinat J, McMahon K, Kuhn S, Schubert F, Schaefer M. Cross-sectional Study of Glutamate in the Anterior Cingulate and Hippocampus in Schizophrenia. Schizophr Bull. 2016;42:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. [DOI] [PubMed] [Google Scholar]
- 66.Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci. 2013;15:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glausier JR, Lewis DA. Mapping pathologic circuitry in schizophrenia. Handb Clin Neurol. 2018;150:389–417. [DOI] [PubMed] [Google Scholar]
- 68.McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016;29:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124:1309–1320. [DOI] [PubMed] [Google Scholar]
- 70.Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. [DOI] [PubMed] [Google Scholar]
- 71.Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci. 2009;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radhu N, Dominguez LG, Greenwood TA, Farzan F, Semeralul MO, Richter MA, Kennedy JL, Blumberger DM, Chen R, Fitzgerald PB, Daskalakis ZJ. Investigating Cortical Inhibition in First-Degree Relatives and Probands in Schizophrenia. Sci Rep. 2017;7:43629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ. Disrupted cortical conductivity in schizophrenia: TMS-EEG study. Cereb Cortex. 2014;24:211–221. [DOI] [PubMed] [Google Scholar]
- 75.Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Altered prefrontal activity and connectivity predict different cognitive deficits in schizophrenia. Hum Brain Mapp. 2015;36:4539–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Homan P, Kindler J, Hubl D, Dierks T. Auditory verbal hallucinations: imaging, analysis, and intervention. Eur Arch Psychiatry Clin Neurosci. 2012;262 Suppl 2:S91–95. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: A meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69–77. [DOI] [PubMed] [Google Scholar]
- 78.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neurosci Biobehav Rev. 2018;89:111–118. [DOI] [PubMed] [Google Scholar]
- 79.He H, Lu J, Yang L, Zheng J, Gao F, Zhai Y, Feng J, Fan Y, Ma X. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: A PRISMA compliant meta-analysis. Clin Neurophysiol. 2017;128:716–724. [DOI] [PubMed] [Google Scholar]
- 80.Kumar N, Vishnubhatla S, Wadhawan AN, Minhas S, Gupta P. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2020;13:840–849. [DOI] [PubMed] [Google Scholar]
- 81.Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–256. [DOI] [PubMed] [Google Scholar]
- 82.Brady RO Jr., Gonsalvez I, Lee I, Ongur D, Seidman LJ, Schmahmann JD, Eack SM, Keshavan MS, Pascual-Leone A, Halko. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am J Psychiatry. 2019;176:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, Fitzgerald PB, Daskalakis ZJ. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–517. [DOI] [PubMed] [Google Scholar]
- 85.Francis MM, Hummer TA, Vohs JL, Yung MG, Visco AC, Mehdiyoun NF, Kulig TC, Um M, Yang Z, Motamed M, Liffick E, Zhang Y, Breier A. Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study. Brain Imaging Behav. 2019;13:852–861. [DOI] [PubMed] [Google Scholar]
- 86.Hasan A, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, Langguth B, Landgrebe M, Eichhammer P, Frank E, Hajak G, Ohmann C, Verde PE, Rietschel M, Ahmed R, Honer WG, Malchow B, Karch S, Schneider-Axmann T, Falkai P, Wobrock T. Cognitive Effects of High-Frequency rTMS in Schizophrenia Patients With Predominant Negative Symptoms: Results From a Multicenter Randomized Sham-Controlled Trial. Schizophr Bull. 2016;42:608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang Y, Guo Z, Xing G, He L, Peng H, Du F, McClure MA, Mu Q. Effects of High-Frequency Transcranial Magnetic Stimulation for Cognitive Deficit in Schizophrenia: A Meta-Analysis. Front Psychiatry. 2019;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas. 2013;25:191–192. [DOI] [PubMed] [Google Scholar]
- 89.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Scientific Advisory B, the Executive Committee of the Grand Challenges on Global Mental H, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 Suppl 1:2–19. [DOI] [PubMed] [Google Scholar]
- 92.Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–486. [DOI] [PubMed] [Google Scholar]
- 95.Draganov M, Vives-Gilabert Y, de Diego-Adelino J, Vicent-Gil M, Puigdemont D, Portella MJ. Glutamatergic and GABA-ergic abnormalities in First-episode depression. A 1-year follow-up 1H-MR spectroscopic study. J Affect Disord. 2020;266:572–577. [DOI] [PubMed] [Google Scholar]
- 96.Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, Rajkowska G. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canali P, Sarasso S, Rosanova M, Casarotto S, Sferrazza-Papa G, Gosseries O, Fecchio M, Massimini M, Mariotti M, Cavallaro R, Smeraldi E, Colombo C, Benedetti F. Shared reduction of oscillatory natural frequencies in bipolar disorder, major depressive disorder and schizophrenia. J Affect Disord. 2015;184:111–115. [DOI] [PubMed] [Google Scholar]
- 98.Voineskos D, Blumberger DM, Zomorrodi R, Rogasch NC, Farzan F, Foussias G, Rajji TK, Daskalakis ZJ. Altered Transcranial Magnetic Stimulation-Electroencephalographic Markers of Inhibition and Excitation in the Dorsolateral Prefrontal Cortex in Major Depressive Disorder. Biol Psychiatry. 2019;85:477–486. [DOI] [PubMed] [Google Scholar]
- 99.Dhami P, Atluri S, Lee JC, Knyahnytska Y, Croarkin PE, Blumberger DM, Daskalakis ZJ, Farzan F. Prefrontal Cortical Reactivity and Connectivity Markers Distinguish Youth Depression from Healthy Youth. Cereb Cortex. 2020;30:3884–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25:247–261. [DOI] [PubMed] [Google Scholar]
- 101.Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, Carvalho AF. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review With Network Meta-analysis. JAMA Psychiatry. 2017;74:143–152. [DOI] [PubMed] [Google Scholar]
- 102.McGirr A, Karmani S, Arsappa R, Berlim MT, Thirthalli J, Muralidharan K, Yatham LN. Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry. 2016;15:85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brunelin J, Jalenques I, Trojak B, Attal J, Szekely D, Gay A, Januel D, Haffen E, Schott-Pethelaz AM, Brault C, Group S, Poulet E. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimul. 2014;7:855–863. [DOI] [PubMed] [Google Scholar]
- 104.Ferrarelli F, Kaskie RE, Graziano B, Reis CC, Casali AG. Abnormalities in the evoked frontal oscillatory activity of first-episode psychosis: A TMS/EEG study. Schizophr Res. 2018. [DOI] [PubMed] [Google Scholar]
- 105.Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A, Bergmann TO, Siebner HR. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage. 2018;185:300–312. [DOI] [PubMed] [Google Scholar]
- 106.Belardinelli P, Biabani M, Blumberger DM, Bortoletto M, Casarotto S, David O, Desideri D, Etkin A, Ferrarelli F, Fitzgerald PB, Fornito A, Gordon PC, Gosseries O, Harquel S, Julkunen P, Keller CJ, Kimiskidis VK, Lioumis P, Miniussi C, Rosanova M, Rossi S, Sarasso S, Wu W, Zrenner C, Daskalakis ZJ, Rogasch NC, Massimini M, Ziemann U, Ilmoniemi RJ. Reproducibility in TMS-EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimul. 2019;12:787–790. [DOI] [PubMed] [Google Scholar]
- 107.Siebner HR, Conde V, Tomasevic L, Thielscher A, Bergmann TO. Distilling the essence of TMS-evoked EEG potentials (TEPs): A call for securing mechanistic specificity and experimental rigor. Brain Stimul. 2019;12:1051–1054. [DOI] [PubMed] [Google Scholar]
- 108.Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS). Clin Neurophysiol. 2002;113:376–382. [DOI] [PubMed] [Google Scholar]
- 109.Maeda F, Pascual-Leone A. Transcranial magnetic stimulation: studying motor neurophysiology of psychiatric disorders. Psychopharmacology (Berl). 2003;168:359–376. [DOI] [PubMed] [Google Scholar]
- 110.Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am J Psychiatry. 2020:appiajp201919070720. [DOI] [PubMed] [Google Scholar]
- 111.Sun Y, Farzan F, Mulsant BH, Rajji TK, Fitzgerald PB, Barr MS, Downar J, Wong W, Blumberger DM, Daskalakis ZJ. Indicators for Remission of Suicidal Ideation Following Magnetic Seizure Therapy in Patients With Treatment-Resistant Depression. JAMA Psychiatry. 2016;73:337–345. [DOI] [PubMed] [Google Scholar]
- 112.Casarotto S, Canali P, Rosanova M, Pigorini A, Fecchio M, Mariotti M, Lucca A, Colombo C, Benedetti F, Massimini M. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Bakker N, Blumberger DM, Daskalakis ZJ, Kennedy SH, Flint AJ, Giacobbe P. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–185. [DOI] [PubMed] [Google Scholar]
- 114.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dinga R, Schmaal L, Penninx B, van Tol MJ, Veltman DJ, van Velzen L, Mennes M, van der Wee NJA, Marquand AF. Evaluating the evidence for biotypes of depression: Methodological replication and extension of. Neuroimage Clin. 2019;22:101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eshel N, Keller CJ, Wu W, Jiang J, Mills-Finnerty C, Huemer J, Wright R, Fonzo GA, Ichikawa N, Carreon D, Wong M, Yee A, Shpigel E, Guo Y, McTeague L, Maron-Katz A, Etkin A. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magnetic stimulation. Neuropsychopharmacology. 2020;45:1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


