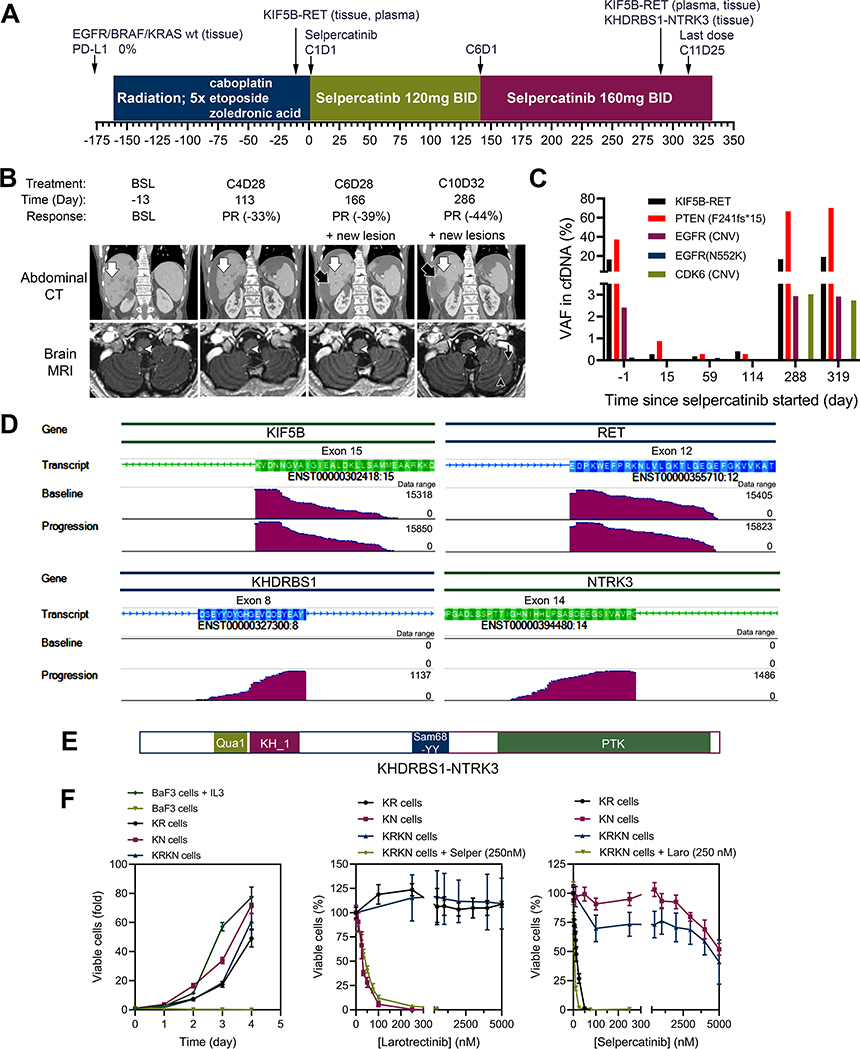
Figure 1.
Treatment history and responses to selpercatinib. (A) Treatment history of a patient with metastatic high-grade neuroendocrine carcinoma of thoracic origin. (B) Serial coronal reformations from a contrast-enhanced abdominal CT (top row) and axial images from contrast-enhanced brain MRI (bottom row) in a 62-year-old man. There are innumerable hepatic and brain lesions, which decrease in size on therapy (examples indicated by white arrow in the liver and white arrowhead in the medulla oblongata). New lesions appear in the liver during cycle 6 (black arrow) and in the brain in cycle 7 (black arrowhead and black thin arrow) and continue to grow on follow-up scans. (C) Levels of variant alleles detected in cfDNA during the course of selpercatinib treatment. (D) Genome browser images illustrating alignments of fusion supporting sequencing reads of cDNA from baseline and progression tissue samples. (E) Schematic presentation of the KHDRBS1-NTRK3 chimeric protein. (F) The parental BaF3 cells required interleukin-3 (IL3) whereas BaF3/KIF5B-RET (KR), BaF3/KDHRBS1-NTRK3 (KN), and BaF3/KIF5B-RET/KDHRBS1-NTRK3 (KRKN) were IL3-independent (left). Cell viability assay of KR, KN, and KRKN cells treated with selpercatinib (Selper), larotrectinib (Laro), or combination of the two drugs.