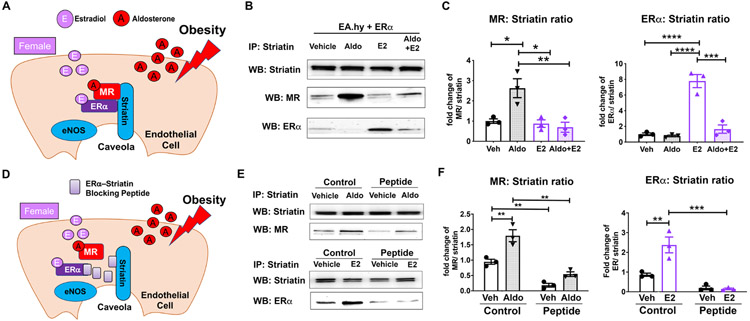
Figure 4. MR and ER Complex with Striatin in Endothelial Cells and Aldosterone Decreases the ERα–Striatin Interaction.
(A) Schematic showing an endothelial cell (EC) exposed to increased aldosterone during obesity, which binds to MR. In females, estradiol is bound to ERα. Both receptors can bind to striatin in EC membrane caveolae to potentiate rapid signaling. (B) Representative immunoblot of EaHY human EC line with stable ERα expression treated with vehicle, 10 nM aldosterone (Aldo), 10 nM estradiol (E2) or E2+Aldo. Cells lysate was immunoprecipitated with anti-striatin antibody and then immunoblotted for striatin, MR, or ERα. (C) The amount of MR or ER bound to striatin was quantified. N=3-4. (D) Schematic showing a cell expressing an ERα mimetic blocking peptide (amino acids 176-253) which prevents ERα-striatin interaction. (D) Representative blots of HEK293 cells overexpressing MR and ERα +/− co-expression of ERα-striatin blocking peptide. Cells were treated with vehicle, Aldo or E2 for 20 minutes and cell lysates were immunoprecipitated with striatin antibody and immunoblotted for striatin, MR, and ERα. (F) The amount of MR or ERα normalized to striatin was significantly decreased with co-expression of the ERα -striatin disrupting peptide when compared to vector plasmid (Control). N=3. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 via one-way ANOVA with Bonferroni post hoc