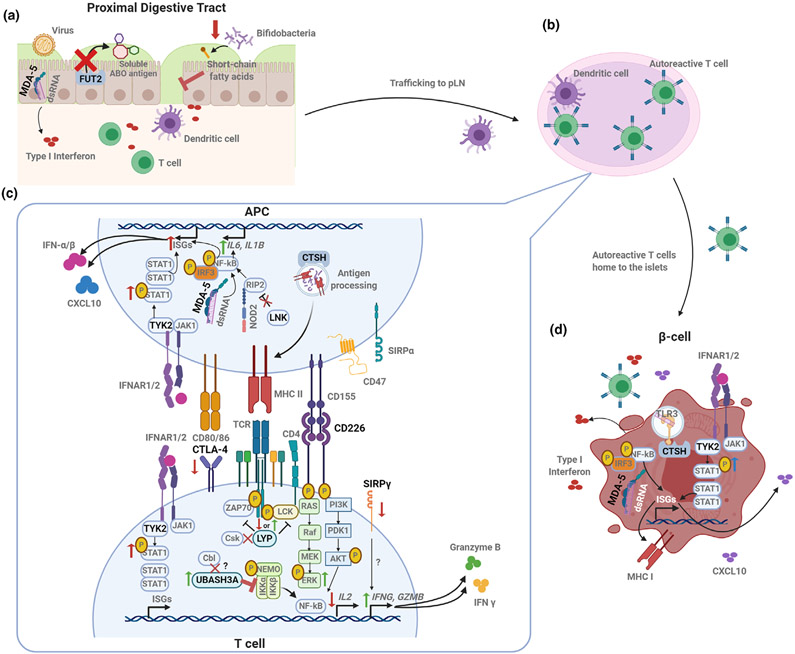
Figure 2.
Coding gene variants converge on signaling and activation pathways to impact autoimmunity. (a) Disruption of FUT2 function and the resulting lack of ABO blood group antigen secretion in the intestinal mucosa can result in impaired barrier function and immunity by increasing susceptibility to some viral infections and altering microbiome composition as well as the microbial metabolites (such as short-chain fatty acids). IFIH1 variants may augment innate responses against enteroviruses. (b) Antiviral or other pro-inflammatory responses in the gut may result in trafficking of APCs to the pancreatic lymph node (pLN), where autoantigen-specific T cells are activated. (c) Activation and function of autoreactive T cells may be exacerbated by T1D-risk variants expressed either in the APC or in the T cell itself. The risk variant of PTPN22 (LYP) abolishes CSK binding, which could result in decreased phosphatase activity and increased TCR signaling. The CTLA4 risk variant results in reduced CTLA4 expression on the T-cell surface, reducing regulation of T-cell activation. Variants in co-stimulatory molecules CD226 and SIRPγ may contribute to pro-inflammatory T-cell skewing by promoting activation of the MAPK/ERK pathway and enhancing the production of inflammatory and cytotoxic molecules, respectively. Treg function may be negatively impacted by a UBASH3A variant, which inhibits NFκB activation and downstream IL-2 production. APCs may contribute to the generation of this pro-inflammatory milieu, as altered LNK function results in enhanced NOD2 signaling, activation of NFκB and production of IL-6 and IL-1β. APC peptide repertoire for presentation may be influenced by a CTSH variant. Variants in TYK2 and IFIH1 may also contribute to innate inflammation through increased induction of IFN stimulated genes (ISGs). (d) Genetic variants may impact β-cell susceptibility to death via sensing of viral or self dsRNA in the case of IFIH1, while a variant within CTSH seems to play a role in TLR3 activation, downstream IFN production and susceptibility to cytokine-induced damage. T1D-associated TYK2 variants may enhance β-cell expression of ISGs, including MHC I and CXCL10. Created with BioRender.