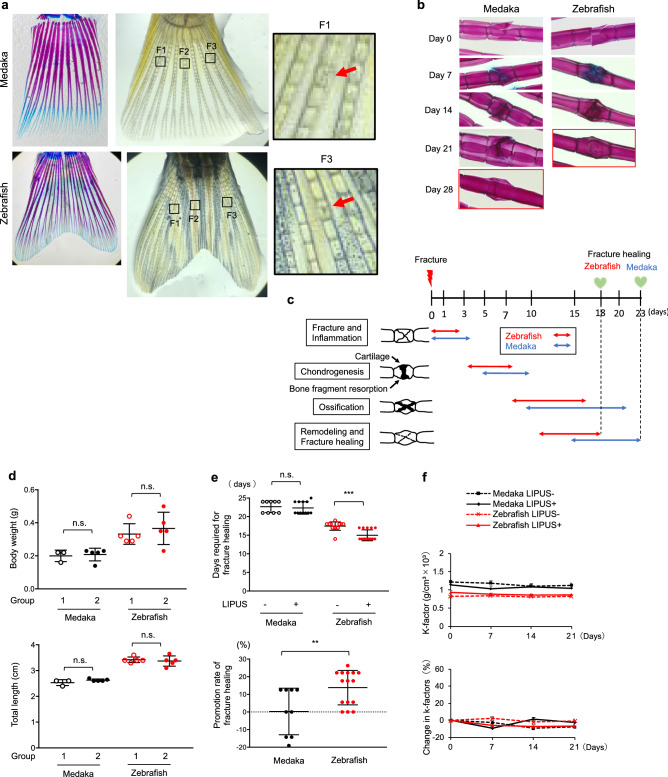
Figure 1.
The effect of LIPUS treatment on fracture healing in medaka and zebrafish (a) Left: Tail bones of medaka and zebrafish stained by Alcian blue and Alizarin red. Middle: In tail bones of zebrafish and medaka, three fractures (F1, F2 and F3) per fish were induced with a scalpel under a microscope. Right: High magnification images of the boxed regions (medaka F1, zebrafish F3) in middle panels. Original magnification 2× (left and middle panel), 10× (right panel). (b) Stained images of the fracture healing process in medaka and zebrafish without LIPUS stimulation. The fracture site was stained with Alcian blue and Alizarin red. Original magnification: 10×. (c) Schematic representation of sequence of fracture healing processes in medaka and zebrafish. Inflammation stage, soft callus formation stage (chondrogenesis), hard callus formation stage (ossification), and remodeling stage are indicated by arrows (Red arrows: zebrafish, Blue arrows: Medaka). (d) The body weight (upper panel) and total length (lower panel) of experimental group of medaka and zebrafish before the LIPUS application (n = 3–5). (e) Upper: Days required for complete fracture healing in LIPUS-treated and untreated medaka and zebrafish. Lower: Promotion rate of fracture healing induced by LIPUS stimulation in medaka and zebrafish (n = 3–5 fishes × 3 fractures, representative data). The time point that the fracture was completely healed was measured in a blinded manner. This experiment was repeated three times with similar results. (f) Upper: The body weight and length of the fish were measured every week, and the condition factor (K-factor; g/cm2 × 103) was calculated at the indicated points. Lower: Percent change in condition factor (n = 3–5). Data are presented as mean ± SEM. p Values were determined using Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.The photographs in (a) and (b) were taken by T. S.