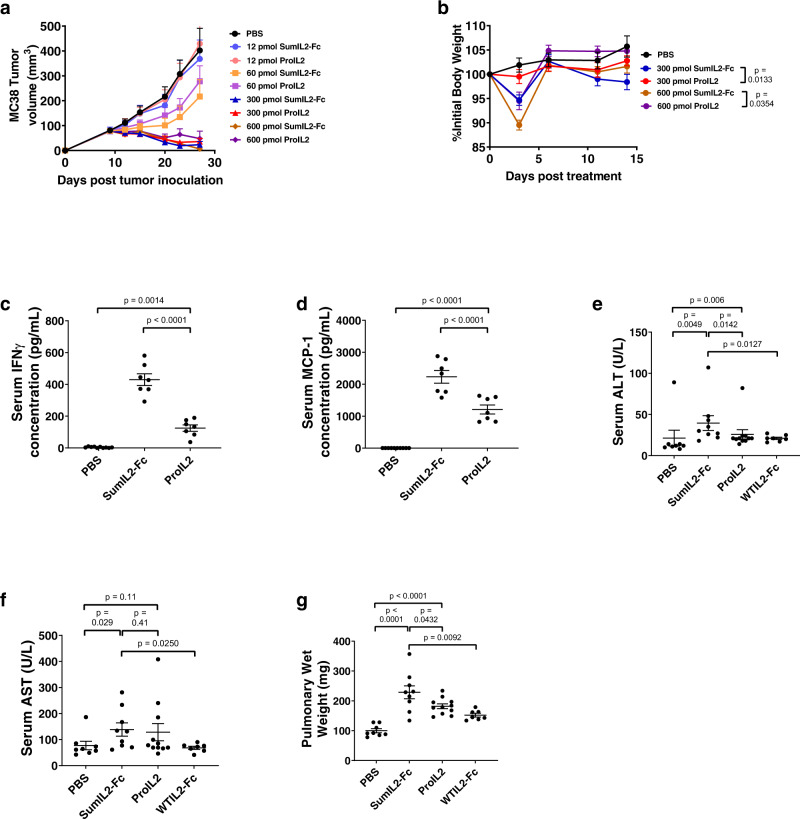
Fig. 4. In vivo antitumor efficacy and toxicity assessment of ProIL2.
a, b MC38 s.c. tumor-bearing mice were injected i.p. with one dose of the labeled treatment on day 9 post tumor inoculation; tumor growth and body weight were measured (n = 2 experiments, total 6 individual mice for 12 pmol treatment groups, 7 for 600 pmol treatment groups, 8 for 60 and 300 pmol treatment groups). c, d B16 s.c. tumor-bearing mice were injected i.p. with PBS, SumIL2-Fc or ProIL2 (120 pmol) one time on day 9 post tumor inoculation. Serum was collected and isolated from mice 24 h post treatment, and Cytometric Bead Array was used to quantify the amount of serum IFN or MCP-1 (n = 2 experiments, total 10 individual mice for PBS treatment group, 7 for other groups). e–g B16 s.c. tumor-bearing mice were injected i.p. with PBS, SumIL2-Fc, ProIL2, or WTIL2-Fc (150 pmol) two times on days 9 and 12 post tumor inoculation. Serum and lungs were collected 48 h after the last treatment. Serum ALT and AST were quantified, along with pulmonary wet weight as described in the “Methods” (n = 3 experiments, total 8 individual mice for PBS treatment group, 9 for SumIL2-Fc, 11 for ProIL2, 7 for WTIL2-Fc). Data represent mean ± s.e.m. Student’s t tests were performed to calculate p values. Source data are provided as a Source Data file.