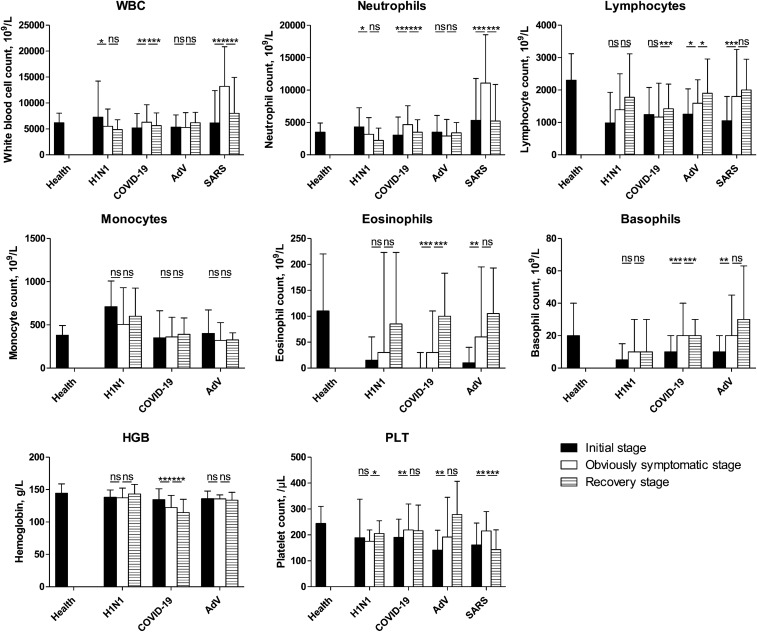
Fig. 5.
Characterization of the four groups of blood tests during the course of COVID-19. Blood test results were collected from patients with pneumonia secondary to COVID-19, SARS, AdV, or H1N1 during hospitalization. The data of the early stage of onset were obtained after performing the test closest to the onset date (i.e., within 3 days after hospitalization); information on stage of disease were obtained on the final test (i.e., within 2 days before discharge). Then, the disease course was divided into three equal periods: the initial, obviously symptomatic, and recovery stages. The data were analyzed using SPSS software version 21. P values between 0.01 and 0.05, 0.001 and 0.01, and 0.0001–0.001 were considered statistically significant (*), very significant (**), and extremely significant (***), respectively. P values > 0.01 were considered not significant