Abstract
Background:
Little data exists regarding the impact of continuous glucose monitoring (CGM) in the primary care management of type 2 diabetes (T2D). We initiated a quality improvement (QI) project in a large healthcare system to determine the effect of professional CGM (pCGM) on glucose management. We evaluated both an MD and RN/Certified Diabetes Care and Education Specialist (CDCES) Care Model.
Methods:
Participants with T2D for >1 yr., A1C ≥7.0% to <11.0%, managed with any T2D regimen and willing to use pCGM were included. Baseline A1C was collected and participants wore a pCGM (Libre Pro) for up to 2 weeks, followed by a visit with an MD or RN/CDCES to review CGM data including Ambulatory Glucose Profile (AGP) Report. Shared-decision making was used to modify lifestyle and medications. Clinic follow-up in 3 to 6 months included an A1C and, in a subset, a repeat pCGM.
Results:
Sixty-eight participants average age 61.6 years, average duration of T2D 15 years, mean A1C 8.8%, were identified. Pre to post pCGM lowered A1C from 8.8% ± 1.2% to 8.2% ± 1.3% (n=68, P=0.006). The time in range (TIR) and time in hyperglycemia improved along with more hypoglycemia in the subset of 37 participants who wore a second pCGM. Glycemic improvement was due to lifestyle counseling (68% of participants) and intensification of therapy (65% of participants), rather than addition of medications.
Conclusions:
Using pCGM in primary care, with an MD or RN/CDCES Care Model, is effective at lowering A1C, increasing TIR and reducing time in hyperglycemia without necessarily requiring additional medications.
Keywords: glucose management, primary care, professional continuous glucose monitoring, quality improvement, type 2 diabetes
Introduction
Lowering glucose to achieve glycemic targets is critical to reduce the risk of complications associated with type 2 diabetes (T2D).1,2 However, a substantial portion of patients with T2D, and the diabetes teams that support them, struggle to achieve glucose targets.3,4 Common barriers include the patient’s lack of education and resources to self-manage their diabetes and ongoing struggle to maintain motivation to adhere to their diabetes regimen. Primary care providers often only have a glycosylated hemoglobin A1c (A1C) to gauge a patient’s overall glycemic management and lack information on specific glucose patterns, making it difficult to confidently recommend therapeutic and lifestyle changes.5 Collectively, this leads to clinical inertia and failure to advance therapy in patients not achieving glycemic targets.
Continuous glucose monitoring (CGM) has the potential to provide patients, providers, and other members of the diabetes care team with critical information to empower patients to achieve better glycemic management.6 By providing more immediate access to pattern-based glucose data throughout the day and night, this technology can help overcome clinical inertia and allow care teams to move beyond A1C as the primary measure of glucose management, to therapy decisions guided by glycemic patterns.7 Real-time CGM (rtCGM) and intermittently scanned CGM (isCGM) have gained considerable acceptance, and in fact are becoming the standard of care, in the management of type 1 diabetes. The 2020 ADA Standards of Medical Care in Diabetes supports use of rtCGM and isCGM in combination with insulin therapy to lower A1C and reduce hypoglycemia in adults with type 2 diabetes who are not meeting glycemic targets.8 While newer technology has allowed rtCGM and isCGM to make inroads in the management of T2D, cost and insurance coverage continue to be limiting factors.
For many healthcare providers, professional CGM (pCGM) allows an accessible option for those unable to manage the commitment or cost of rtCGM or isCGM, and individuals managed less intensively who may benefit from insights into the impact of medication and lifestyle choices on glycemic patterns. Blinded pCGM (the data are not viewable by the patient until the device is removed), is an American Diabetes Association Grade E (expert opinion) recommendation to help “in identifying and correcting patterns of hyper- and hypoglycemia in people with type 1 diabetes and type 2 diabetes”.8
Advances in pCGM technology have resulted in availability of CGM devices with greater ease of use: easier application, no need for calibration, disposable sensors not requiring sterilization between patient uses, and less interference by compounds like acetaminophen. These advances have made the use of pCGM in primary care settings significantly more feasible. International Diabetes Center developed the Ambulatory Glucose Profile (AGP) Report to make interpretation of complex glucose data and glycemic patterns more straightforward and clinically meaningful. AGP Reports have become the standard way of representing CGM data.9-13 Abbott Diabetes Care has adapted the AGP Report and included it in the LibreView report.
While use of pCGM in the primary care setting has expanded to some degree, lack of established work flows and difficulty in implementing rapidly changing technology on a wider scale in primary care, as well as lack of familiarity with the technology and interpretation of AGP Reports, have all continued to be barriers to wider use in a primary care setting. This is especially concerning given the steady rise in the number of patients with diabetes and declining number of endocrinologists available to treat them. We initiated a quality improvement (QI) project in a large integrated health care system to determine the effect of pCGM on glucose management in a team-based primary care setting. Change in glucose management was determined by change in A1C and time in range (TIR) metrics. The goal was to develop best practices and standard work for integrating this technology into established primary care work flow with minimal disruption and maximal impact on improving diabetes management. We evaluated 2 deployment models within our existing primary care team-based diabetes management framework: an MD Care Model and an RN/Certified Diabetes Care and Education Specialist (CDCES) Care Model.
Methods
This QI project was started in September, 2017 and completed in the fall of 2018. It was conducted in 2 internal medicine departments at Park Nicollet Clinic, a large, integrated multi-group practice in the Minneapolis, Minnesota metropolitan area. Workflows were created to use pCGM in a traditional clinic-based MD-LPN or MA Care Model, as well as a more team-based RN/CDCES Care Model. In this model, the RN/CDCES may also have referred the participant to a registered dietitian nutritionist for additional lifestyle behavior changes and/or consulted with a supervising MD if required (e.g., addition of noninsulin therapy or addition of a new insulin).
We sought to enroll established participants that were diagnosed with T2D for at least 1 year, not meeting glycemic goals defined as most recent A1C ≥7.0% and <11.0% and willing to wear a pCGM device. Participants enrolled in this project were not limited by type of T2D management regimen. Professional CGM data was collected with Abbott Freestyle Libre Pro CGM. Participants were encouraged to continue their usual use of blood glucose monitoring (BGM) while using blinded pCGM, and were encouraged to make notes regarding diet and exercise during the period in which they wore the pCGM for reference at the time of follow-up visit. The HealthPartners Institutional Review Board waved need for approval since this was a QI project using approved diabetes therapies and technologies.
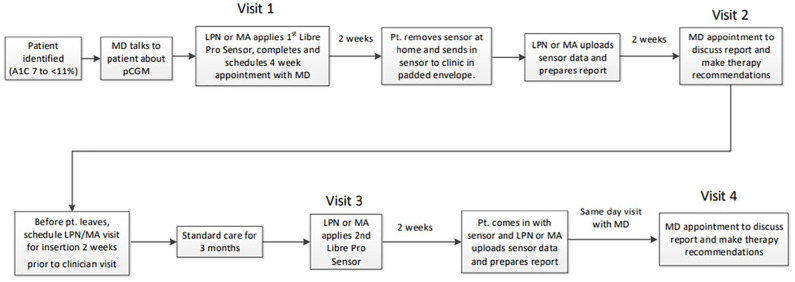
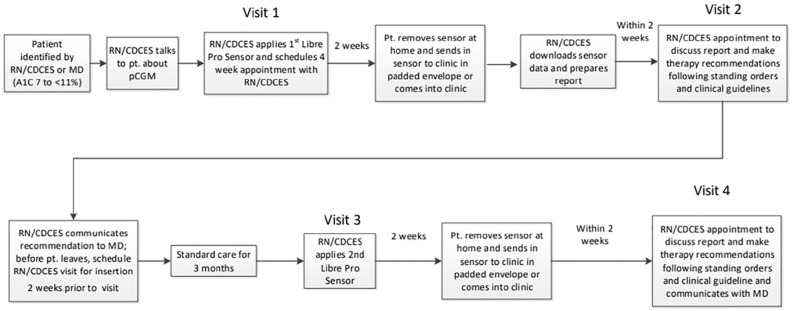
Work flows were created to allow nurses/MA’s or CDCES’s to apply a professional CGM sensor after discussion of risks versus benefits (Figures 1 and 2). A follow-up visit with the physician or CDCES was scheduled at the time of sensor placement. After pCGM wear (optimally for 14 days) the sensor was either returned to the clinic by mail, or returned to the clinic by the participant. Staff then downloaded sensor data for use at a follow-up visit with the clinician or CDCES, typically 3 to 4 weeks after placement of the sensor.
Figure 1.
MD Care Model pCGM primary care clinic process workflow.
Figure 2.
RN/CDCES Care Model pCGM primary care clinic process workflow.
After collection of baseline A1C and pCGM data, participants met with an MD or RN/CDCES (in consultation with MD) to review the pCGM data and AGP Report. Shared decision making was used to modify lifestyle and medication regimen. Participants returned to clinic in 3 to 6 months for follow-up A1C and clinic visit. A subset of participants underwent repeat pCGM at the time of their follow-up visit.
Results
Sixty-eight participants agreed to be part of the QI project and their baseline demographic and clinical characteristics are shown in Table 1. Participants had a long duration of diabetes, and the majority were treated with a combination of insulin and noninsulin therapies, likely related to being drawn from established internal medicine practices.
Table 1.
Baseline Demographic and Clinical Characteristics.
| Mean age | 61.6 years |
| Mean duration of diabetes | 15 years |
| Mean A1C | 8.8% |
| Average number of medications | 2.7 |
| Prescribed medication (%): | |
| Background (basal) insulin | 81 |
| Mealtime insulin | 57 |
| Metformin | 62 |
| Sulfonylurea | 21 |
| GLP-1 receptor agonist | 21 |
| SGLT2 inhibitor | 9 |
| DPP-4 inhibitor | 4 |
| Pioglitazone | 3 |
The use of pCGM resulted in improvement in glucose management. The mean A1C for the entire cohort of 68 participants was reduced from a baseline of 8.8% ± 1.2% to 8.2% ± 1.3% (P=0.006) after pCGM. At baseline, 22% of participants had an A1C <8% and 40% had an A1C >9%. After pCGM 52% of participants had an A1C <8% and 24% had an A1C >9%. Change in A1C was determined for each of the 2 Care Models. Participants treated using the MD Care Model (n=20) had mean reduction of A1C of 1 percentage point with most patients experiencing a reduction in A1C (see Figure 3). While A1C decreased in most participants, 4 out of the 20 participants’ A1C rose slightly (0.1-0.4 percentage points) and 1 participant who started with an A1C of 10% increased to >13%. Participants treated using the RN/CDCES Care Model (n=48) had mean reduction of A1C of 0.6 percentage points with most patients experiencing a reduction in A1C (see Figure 4). One participant started with an A1C of 5.7% (outside the inclusion criteria) that increased to 6.1% showing that the RN/CDCES identified a patient who would potentially benefit from pCGM to determine if hypoglycemia was a concern.
Figure 3.
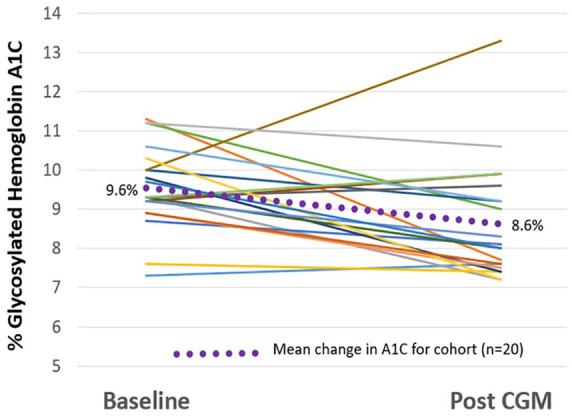
Change in A1C in MD Care Model.
Figure 4.
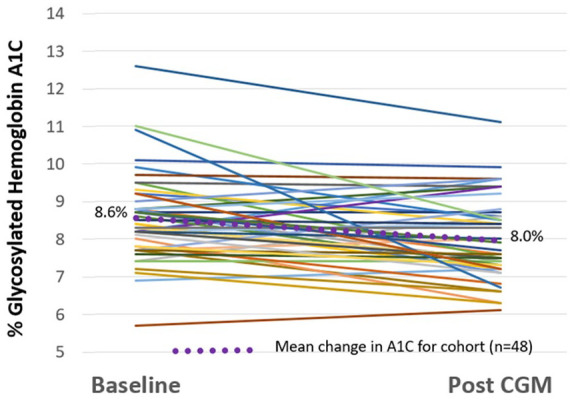
Change in A1C in RN/CDCES Care Model.
As part of the QI project, baseline pCGM data from the first sensor wear was compared to data from the second sensor wear to determine the impact on glucose management. Thirteen out of the 20 participants (65%) in the MD Care Model and 24 out of 48 participants (50%) in the RN/CDCES model had complete data defined as baseline and 3 to 6 month follow-up pCGM data. Analysis of participant’s pCGM data where baseline and follow-up pCGM data were available is summarized in Table 2. TIR increased in both models with a greater increase noted in the MD Care Model. The time below range (<54 mg/dL and <70 mg/dL) increased modestly in the RN/CDCES Care Model and was more pronounced in the MD Care Model increasing from 1.0% to 2.1% in the very low glucose range (<54 mg/dL). Time above range (>180 mg/dL) decreased in both groups with no significant changes to percent coefficient of variation baseline compared to follow-up pCGM.
Table 2.
Time in Ranges CGM Metrics.
| Metric | MD Care Model (n=13) |
RN/CDCES Care Model (n=24) | ||
|---|---|---|---|---|
| Baseline (%) | Follow-up pCGM (%) | Baseline (%) | Follow-up pCGM (%) | |
| % Time <54 mg/dL | 1.0 | 2.1 | 1.0 | 1.2 |
| % Time <70 mg/dL | 2.9 | 5.5 | 2.5 | 3.4 |
| % TIR 70-180 mg/dL | 40.8 | 58.5 | 53.7 | 58.6 |
| % Time >180 mg/dL | 56.2 | 36.0 | 43.9 | 38.0 |
| % Coefficient of variation (CV) | 32.8 | 32.3 | 31.9 | 31.1 |
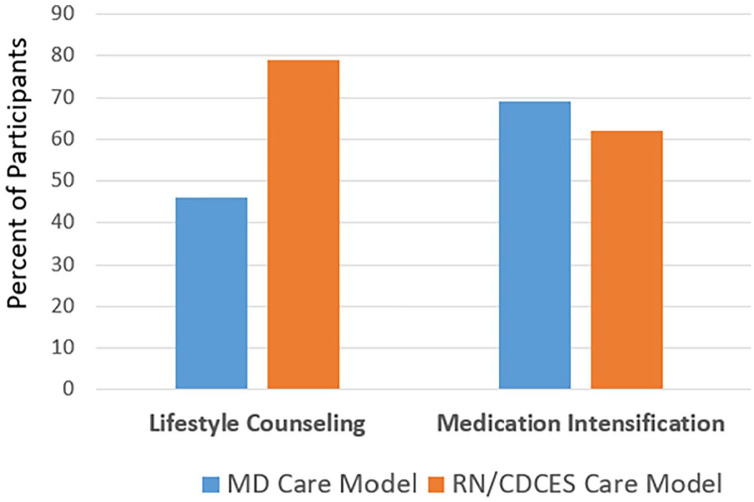
Analysis of the types of interventions that occurred in the subset of 37 patients with complete baseline and follow-up pCGM was determined. As shown in Figure 5, the physicians focused more on medication intensification and less on lifestyle counseling. The RN/CDCES focused more on lifestyle counseling than medication intensification with nearly 80% of patients receiving lifestyle counseling based on pCGM, yet their medication intensification was only slightly less than the MD model.
Figure 5.
Interventions after pCGM.
Analysis of the average number of medications before and after pCGM and was conducted on the subset of 37 patients with complete baseline and follow-up data. As shown in Figure 6, the number of medications did not change significantly.
Figure 6.
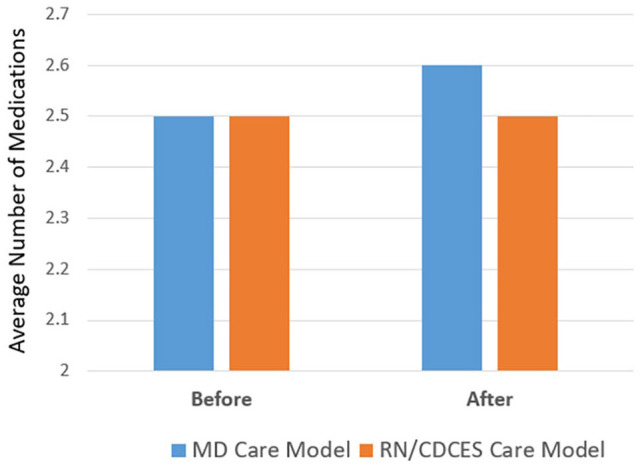
Number of medications before and after pCGM.
Discussion
This QI project sought to evaluate the impact of pCGM usage on glycemic management in a real world primary care setting, using 2 different care models. The first was a traditional physician-LPN/MA team in the clinic setting (MD Care Model). The second was a more team-based model utilizing existing RN certified diabetes care and education specialists working under the supervision of a primary care physician to facilitate collection and interpretation of pCGM data (RN/CDCES Care Model). The RN/CDCES may have also referred patients to a registered dietitian nutritionist for lifestyle and nutrition education. Our intent was to both evaluate the feasibility of using pCGM as a tool to optimize glycemic management for patients who do meet glycemic goals, and to evaluate the real-world barriers and facilitators to the practical application of this technology in a primary care setting.
Over the course of this QI project, 68 participants who completed an initial single cycle of pCGM wear, followed by a follow-up visit in 3 to 6 months for re-evaluation of A1C, were able to improve their A1C by an average of 0.6 percentage points. The improvement resulted from lifestyle and medication adjustments agreed upon through shared-decision making after using blinded pCGM for up to 2 weeks. A second pCGM was recorded prior to this follow-up visit in a smaller subset who participated in a second period of pCGM wear. In this subset (n=37), average time in range (TIR 70-180 mg/dL) and time above range (>180 mg/dL) improved in both the MD Care Model and RN/CDCES Care Model, but at the cost of increased time in hypoglycemia. The increase in both time in range, and time in hypoglycemia, was more prominent in the MD Care Model, suggesting a difference in the intensity of medication intervention between the groups. Several individuals in both groups had a modest rise in A1C, and 1 individual in the MD group had a significant rise in A1C reinforcing that CGM technology is not a panacea that ensures success, but is rather a tool that has the potential to improve glycemic metrics for many.
Notable also is that the improvement in glycemic management was accomplished by a combination of lifestyle counseling and medication intensification, rather than an increase in the number of medications, which remained stable. The primary medication intensification was an increase in insulin doses showing that pCGM can provide useful information to guide the diabetes team in making insulin adjustments. It was clear that having pCGM data and AGP Reports were useful in both models to guide lifestyle modifications (e.g., healthy eating, physical activity, medication adherence). As might be expected, participants in the RN/CDCES Care Model received more lifestyle counseling to help the patient manage their diabetes than in the MD Care Model yet still making almost as many medication adjustments as the MD Care Model; the physicians tended to focus more on medication intensification with less focus on lifestyle management. Overall, this supports the concept that pCGM and AGP Reports are utilized successfully by the various members of the diabetes team to facilitate both lifestyle and medication changes, albeit with a slightly different focus.
Practical (subjective) learnings included that the work flows (Figures 1 and 2), once established, function reasonably well, and were able to be integrated into standard rooming practice. Technology barriers were encountered with the downloading of data, which was initially accomplished via software based on several departmental computers, but then transitioned to “cloud-based” access, which allowed data access from multiple work stations, as well as previously collected data, for comparison. Access to cloud-based systems existing outside the health system firewall required work with health system information technology personnel to overcome firewall barriers and allow access to necessary drivers.
The pCGM sensors were generally well tolerated by the participants, with minimal issues with skin irritation. Sensor adhesion was problematic for some individuals; adhesive overlays and barrier skin protectant (individually packaged skin wipes) were helpful in optimizing adhesion.
The glycemic outcomes observed in this project compare similarly to data reported in the first 6 months of a larger study conducted subsequent to our project, the GP-Osmotic study.14 Whether our glycemic outcomes would have been sustained, or would have fallen off as did the outcomes in GP-Osmotic is not known.
It was felt that further clinician education on the use of the AGP Report to titrate medication, as well as promote lifestyle discussions, would have been helpful. Based on this feedback we subsequently conducted training on using AGP Reports to facilitate lifestyle, noninsulin and insulin therapy changes in our health system. Clinical targets for glycemic metrics have also been established subsequent to this project15 and this standardization should allow improved consistency in the management of CGM data and AGP Reports.
The strength of this project is that it was conducted in a real world setting, using available personnel, and established the feasibility of using pCGM technology in a primary care setting. It integrated 2 different models of care and multiple members of the diabetes care team including MD, LPN and MA staff, RN/CDCES, and occasionally a registered dietitian nutritionist to provide additional lifestyle education. The workflows and models of care could potentially be implemented in other healthcare systems. Limitations include that it lacked the rigor and consistent follow-up typical of a research study, which may limit generalizability of the glycemic results. A second pCGM sensor wear was integrated into the workflow for both care models yet only slightly more than 50% of participants added an additional clinic visit to have a second sensor applied. Also, CGM technology is not completely accurate and the Libre Pro CGM is less accurate in the hypoglycemic range. It may inaccurately indicate (over report) hypoglycemia when the individual is not actually experiencing low blood glucose.16 Future considerations to optimize the use of this technology in typical primary care settings may include further clinician training in the interpretation of AGP Reports, as well as further optimization of work flows to improve consistent patient follow up to avoid therapeutic inertia and degradation of glycemic impact over time. Additionally, integration of CGM data more directly into electronic medical record formats would allow simplification of workflows and optimized access to data throughout the care team.
Conclusions
Using pCGM in a primary care setting with either an MD or RN/CDCES Care Model, is effective at lowering A1C, increasing TIR (70-180 mg/dL) and reducing time above target (>180 mg/dL) without increasing average number of diabetes medications. pCGM did not reduce hypoglycemia and caution must be exercised in medication (insulin) intensification to avoid increased time below range (<54 mg/dL and <70 mg/dL).
Acknowledgments
The authors would like to thank Dr. Deborah Mullen for assistance in statistical evaluation. This project could not have been completed without the dedicated work of Dr. Steven Reed, Dr. Ange Krause, Cheryl Beech RN, CDCES, Suellen Hanson RN, CDCES, Nancy Destache, RN, CDCES, Libby Johnson, RDN, LD, CDCES, and Janet Lima MPH, RN, CDCES who helped refine workflows and provided clinical care essential to completion of this QI project. Julie Cashman, RN, and Jennifer Hoppe, RN, helped in the development of clinical nursing workflows.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; A1C, glycosylated hemoglobin A1c; BGM, blood glucose monitoring; CDCES, Certified Diabetes Care and Education Specialist; CGM, continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; MA, Medical Assistant; pCGM, professional continuous glucose monitoring; QI, quality improvement; rtCGM, real-time continuous glucose monitoring; TIR, time in range; T2D, type 2 diabetes
Authors’ Note: Portions of this work have been previously presented in abstract and poster form at the 2019 American Diabetes Association Scientific Meeting.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GDS has received unrestricted educational grants from Abbott Diabetes Care and Medtronic and is on the scientific advisory board for Merck. GDS receives no personal income from these relationships and all funds and honorariums go directly to support the mission of the non-profit International Diabetes Center.
RMB has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur Corporation, DexCom, Hygieia, Insulet, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi and United Healthcare. His technology research is funded in part by NIH/NIDDK. RMB’s employer, non-profit HealthPartners Institute, contracts for his services and no personal income goes to RMB.
JLD reports no conflicts of interest.
MLJ has received research support from Abbott Diabetes Care, CeQur Corporation, DexCom, Hygieia, Insulet, JDRF, Lilly, Medtronic, NIH/NIDDK, Novo Nordisk, and Sanofi. MLJ’s employer, non-profit HealthPartners Institute, contracts for her services and no personal income goes to MLJ.
TWM is personally involved in a number of industry funded or sponsored research studies with Dexcom, Medtronic, Abbott Diabetes Care, Insulet, Novo-Nordisk, and Eli Lilly. His employer, non-profit HealthPartners Institute, contracts for his services and no personal income goes to TWM.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Abbott Diabetes Care
ORCID iDs: Gregg D. Simonson  https://orcid.org/0000-0001-5561-2547
https://orcid.org/0000-0001-5561-2547
Richard M. Bergenstal  https://orcid.org/0000-0002-9050-5584
https://orcid.org/0000-0002-9050-5584
Thomas W. Martens  https://orcid.org/0000-0002-9858-2138
https://orcid.org/0000-0002-9858-2138
References
- 1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 2. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med. 2019;179(10):1376-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425-1432. [DOI] [PubMed] [Google Scholar]
- 5. Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabetes Ther. 2019;10(3):853-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergenstal RM. Continuous glucose monitoring: transforming diabetes management step by step. Lancet. 2018;391:1334-1336. [DOI] [PubMed] [Google Scholar]
- 7. Carlson AL, Criego AB, Martens TW, Bergenstal RM. HbA1c: the glucose management indicator, time in range, and standardization of continuous glucose monitoring reports in clinical practice. Endocrinol Metab Clin North Am. 2020;49(1):95-107. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S77-S88. [DOI] [PubMed] [Google Scholar]
- 9. Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal RM. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21(suppl 2):S217-S225. [DOI] [PubMed] [Google Scholar]
- 10. agpreport.org [internet]. International Diabetes Center. Ambulatory Glucose Profile. Accessed November 25, 2020. http://www.agpreport.org/agp/about
- 11. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S66-S76. [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(suppl 1):S71-S80. [DOI] [PubMed] [Google Scholar]
- 13. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15(3):198-211. [DOI] [PubMed] [Google Scholar]
- 14. Furler J, O’Neal D, Speight J, et al. Use of professional-mode flash glucose monitoring, at 3-month intervals, in adults with type 2 diabetes in general practice (GP-OSMOTIC): a pragmatic, open-label, 12-month, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8(1):17-26. [DOI] [PubMed] [Google Scholar]
- 15. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the freestyle libre pro flash Continuous Glucose Monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43(11): 2730-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]