Abstract
Aims
This study aimed to determine the effects of sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) in heart failure with reduced ejection fraction (HFrEF), compare the effect of SGLT2i with angiotensin receptor neprilysin inhibitor (ARNI), and find whether combination of SGLT2i and ARNI is better than monotherapy.
Methods and results
Embase, Medline, and Cochrane Central Registry of Controlled Trials were searched for randomized controlled trials evaluating SGLT2i or ARNI in HFrEF. And a total of six trials were included. SGLT2i was found to significantly reduce the risk of cardiovascular death or hospitalization for heart failure by 27% [hazard ratio (HR) 0.73, 95% confidence interval (CI) 0.67–0.80], hospitalization for heart failure by 31% (HR 0.69, 95% CI 0.62–0.77), cardiovascular death by 16% (HR 0.84, 95% CI 0.74–0.95), and all‐cause death by 16% (HR 0.84, 95% CI 0.75–0.94) in HFrEF only with a statistically higher risk of genital infection (risk ratio (RR) 2.78, 95% CI 1.46–5.29). The reduction in cardiovascular death or hospitalization for heart failure was of similar magnitude in patients with or without diabetes mellitus (HR 0.71, 95% CI 0.64–0.80 vs. HR 0.75, 95% CI 0.65–0.87) using SGLT2i. Indirect treatment comparison showed that SGLT2i and ARNI had similar effects on primary outcome (HR 0.93, 95% CI 0.82–1.06). And combination of SGLT2i and ARNI achieved a better prognosis performance (HR 0.68, 95% CI 0.53–0.89) compared with ARNI monotherapy.
Conclusions
SGLT2i could safely reduce cardiovascular death or hospitalization for heart failure in HFrEF regardless of diabetes mellitus status. SGLT2i and ARNI demonstrate similar effects, while combination of SGLT2i and ARNI results in a better cardiovascular protective effect.
Keywords: Sodium‐glucose cotransporter‐2 inhibitor, Angiotensin receptor neprilysin inhibitor, Heart failure with reduced ejection fraction, Meta‐analysis
Introduction
Heart failure is defined as cardiac systolic and diastolic dysfunction caused by various factors and characterized by reduced cardiac output and pulmonary and systematic congestion. It has become the most common complication of different heart diseases, such as coronary heart disease, valvular heart disease, and cardiomyopathy. It is also the cause of many disabilities and deaths worldwide. Lots of effort have been devoted to seeking the optimal treatment for heart failure, which could relieve symptoms and improve prognosis. Diuretics and digitalis, which can reduce the cardiac load and increase contractility of the heart muscle, respectively, are commonly used in patients with heart failure to relieve their congestive symptoms. Beta‐blockers, mineralocorticoid receptor antagonists, and angiotensin‐converting enzyme inhibitors (ACEI)/angiotensin receptor inhibitors (ARB) have been used due to their effects on both symptom and prognosis improvement, including enhancing the quality of life, improving ventricular remodelling, and reducing fatality and hospitalization rates. 1 , 2 , 3 For a time, the combined application of these drugs has been considered to be the best treatment for patients with heart failure. 4 , 5 In recent years, two newly emerged drugs, sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) and angiotensin receptor neprilysin inhibitor (ARNI), have shown better cardiovascular protective effects in patients with heart failure compared with conventional treatments. 6 , 7
ARNI, which is a combined preparation of valsartan and sacubitril, has been shown to have greater haemodynamic and neurohormonal effects in patients with heart failure. However, ARNI has not demonstrated any significant prevention effects on cardiovascular events in patients with preserved ejection fraction (EF). 8 In heart failure with reduced ejection fraction (HFrEF), ARNI was superior to ACEI in reducing the risk of cardiovascular death or hospitalization for heart failure. 9 SGLT2i is a new hypoglycaemic drug that has been found to have a pleiotropic function in addition to lowering serum glucose in patients with diabetes mellitus (DM), including lowering blood pressure, reducing cardiovascular risk, and protecting kidney function. 10 , 11 A previous meta‐analysis that pooled three large‐scale trials, EMPA‐REGOUTCOME, DECLARE‐TIMI 58, and CANVAS, has found that SGLT2i usage in patients with heart failure history significantly reduced the occurrence of cardiovascular death and hospitalization for heart failure. 12 However, the definition of heart failure history varied among the three trials, and stratified heart failure results were unclear. Whether patients with confirmed HFrEF could also benefit from SGLT2i needed further exploration. However, much evidence for cardiovascular event prevention by SGLT2i came from patients with DM, and it is unclear whether it has the same protective effect in patients with HFrEF without DM. Then whether SGLT2i or ARNI is more effective in reducing cardiovascular event risk has not been investigated yet. Lastly, whether combination usage of these two drugs would achieve better outcomes than a monotherapy is also unclear. The goal of the present systematic review and meta‐analysis was to explore these questions in detail, relying upon available evidence.
Methods
Search strategy and selection criteria
Electronic databases, including Embase, PubMed, and Cochrane Central Registry of Controlled Trials (CENTRAL), were searched for randomized controlled trials (RCTs) that evaluated the effects of SGLT2i or ARNI in patients with HFrEF published up to 1 September 2020. The inclusive criteria were as follows: (i) clinical RCTs; (ii) patients with HFrEF with left ventricular EF of <45%; and (iii) trials providing primary outcome data (cardiovascular death or hospitalization for heart failure). The search items included the following: valsartan/sacubitril, angiotensin receptor‐neprilysin inhibitor, LCZ696, sodium‐glucose cotransporter‐2 inhibitors, empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, ipragliflozin, luseogliflozin, tofogliflozin, sotagliflozin, heart failure, reduced ejection fraction, cardiovascular death, and hospitalization for heart failure. A highly sensitive search strategy for identifying RCTs was also used. The detailed search strategies are illustrated in Supporting Information, Appendix S1 . Three investigators independently reviewed all of the database searches and retrieved the eligible trials by viewing the title and abstract, followed by the full text. Disagreements were resolved by discussion.
Data extraction and quality assessment
Two reviewers independently extracted detailed information from the trials, which included study and patient population characteristics, definition of HFrEF, SGLT2i and ARNI type, comparator type, and clinical outcomes. The extracted data were compared and disagreements were resolved either through discussion or by involving a third party to achieve consensus when necessary. Bias risk evaluation tools from the Cochrane Collaboration for assessment of risk of bias in RCTs were used, which contained the following items: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias.
Statistical analysis
Dichotomous data were computed using hazard ratio (HR) or risk ratio (RR) and 95% confidence interval (CI). The traditional head‐to‐head meta‐analyses were calculated using the inverse variance method with a fixed model. Heterogeneity was assessed using Cochrane Q statistic and Higgins and Thompson I 2. Heterogeneity was considered to be low if I 2 = 25%, moderate if I 2 = 50%, or high if I 2 = 75%. Comparisons without direct evidence were analysed using the indirect treatment comparison (ITC) with a fixed model. STATA/MP software Version 14.0 (StataCorp, College Station, Texas) was used for both traditional direct head‐to‐head comparison and indirect comparison result calculation. The use of P < 0.05 was considered to indicate statistical significance.
Systematic review registration
We have registered in PROSPERO, and the registered number is CRD 42020205920.
Results
Eligible studies and subject characteristics
The study selection flow chart is presented in Figure 1 . A total of 566 potential trials were identified from electronic databases. A total of six RCTs (DECLARE‐TIMI 58, DAPA‐HF, EMPEROR‐HF, SOLOIST‐WHF, PARADIGM‐HF, and PIONEER‐HF) were included in the mixed meta‐analysis, 6 , 7 , 13 , 14 , 15 , 16 of which four trials were directly designed to evaluate the efficacy of SGLT2i in patients with HFrEF and two to investigate the efficacy of ARNI. And the data of DECLARE‐TIMI 58 and PIONEER‐HF were extracted from the second analyses and SOLOIST‐WHF from a post hoc. The definition of HFrEF included an EF of ≤40% in four trials, while the remaining trial was defined by EF < 45%. Detailed baseline characteristics information for the included trials is presented in Table 1 . All trials were in low risk bias. Risk bias assessment summary results were shown in Supporting Information, Figure S1.
Figure 1.
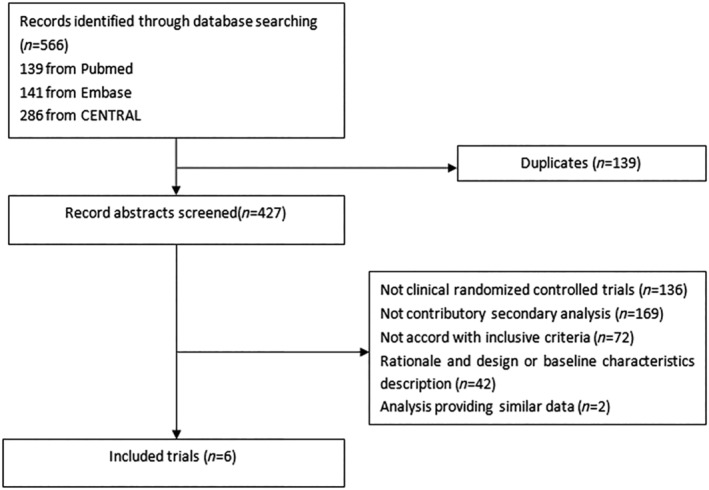
Flow diagram of included trials.
Table 1.
Baseline characteristics of included trials
| DECLARE‐TIMI 58 16 | DAPA‐HF 7 | EMPEROR‐HF 14 | SOLOIST‐WHF a , 15 | PARADIGM‐HF 6 | PIONEER‐HF 13 | |
|---|---|---|---|---|---|---|
| Comparison | Dapagliflozin 10 mg daily vs. placebo | Dapagliflozin 10 mg daily vs. placebo | Empagliflozin 10 mg daily vs. placebo | Sotagliflozin 200 mg once daily (with a dose increase to 400 mg, depending on side effects) vs. placebo | Sacubitril/valsartan titrated to 200 mg twice daily vs. enalapril titrated to 10 mg twice daily | LCZ696 200 mg twice daily vs. enalapril 10 mg twice daily |
| Publication year | 2019 | 2019 | 2020 | 2020 | 2014 | 2018 |
| HFrEF definition | EF < 45% | EF ≤ 40% | EF ≤ 40% | <40% | EF ≤ 40% | EF ≤ 40% |
| Median left ventricular ejection fraction (%) | 38 | 31.1 | 27.4 | NA | 29.5 | 24.5 |
| Median follow‐up | 50.4 months | 18.2 months | 16 months | 9 months | 27 months | 8 weeks |
| Number of participants | 671 | 4744 | 3730 | 725 | 8399 | 881 |
| Median age (years) | 63 | 66.3 | 64.6 | NA | 63.8 | 62 |
| Sex, no. (%) | ||||||
| Men | 565 (84.2) | 3635 (76.6) | 2837 (76.1) | NA | 6567 (78.2) | 635 (72.1) |
| Women | 106 (15.8) | 1109 (23.4) | 893 (23.9) | NA | 1832 (21.8) | 246 (27.9) |
| NYHA class, no. (%) | ||||||
| I | 217 (32.4) | 0 | 0 | NA | 389 (4.6) | 9 (1) |
| II | 378 (56.4) | 3203 (67.5) | 2800 (75.1) | NA | 5919 (70.5) | 222 (25.2) |
| III | 73 (10.8) | 1498 (31.6) | 910 (24.4) | NA | 2018 (24) | 552 (62.7) |
| IV | 0 | 43 (0.9) | 20 (0.5) | NA | 60 (0.7) | 75 (8.5) |
| Not assessed | 3 (0.5) | 0 | 0 | NA | 13 (0.2) | 23 (2.6) |
| Median NT‐proBNP (pg/mL) | NA | 1437 | 1907 | NA | 1612 | 2709 |
| Medical history, no. (%) | ||||||
| Atrial fibrillation | NA | 1818 (38.3) | 1369 (36.7) | NA | 3091 (36.8) | NA |
| Diabetes mellitus | 671 (100) | 1983 (41.8) | 1856 (49.8) | NA | 2907 (34.6) | NA |
| Main clinical outcomes | Composite of cardiovascular death or hospitalization for heart failure; cardiovascular death; hospitalization for heart failure; all‐cause mortality | Composite of worsening heart failure or death from cardiovascular causes; hospitalization for heart failure; cardiovascular death; composite of hospitalization for heart failure or cardiovascular death; death from any cause | Composite of cardiovascular death or hospitalization for heart failure; hospitalization for heart failure; cardiovascular death; death from any cause | Occurrence of either death from cardiovascular causes or hospitalization for heart failure, as described in the trial protocol | Death from cardiovascular causes or a first hospitalization for heart failure; death from cardiovascular causes; first hospitalization for worsening heart failure; death from any cause | Composite of major clinical events; death from any cause; rehospitalization for heart failure; composite of death and rehospitalization for heart failure |
EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; NA, not applicable; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Post hoc.
SGLT2i efficacy evaluation in patients with HFrEF
Meta‐analysis was conducted to perform SGLT2i efficacy evaluation in patients with HFrEF using data from four trials, including DECLARE‐TIMI 58, DAPA‐HF, EMPEROR‐HF, and SOLOIST‐WHF. The pooled results demonstrated that SGLT2i significantly reduced the risk of composite cardiovascular death or hospitalization for heart failure outcomes by 27% (HR 0.73, 95% CI 0.67–0.80). For individual outcomes, only the former three trials provided needed information, and the results showed that SGLT2i reduced the occurrence of hospitalization for heart failure by 31% (HR 0.69, 95% CI 0.62–0.77) and cardiovascular death by 16% (HR 0.84, 95% CI 0.74–0.95). The SGLT2i group also demonstrated a lower risk of all‐cause death (HR 0.84, 95% CI 0.75–0.94). Because moderate heterogeneity presented in cardiovascular death and all‐cause death analyses, a random model was used to compute these data meanwhile. The results also showed a similar SGLT2i prevention effect on cardiovascular death (HR 0.82, 95% CI 0.67–0.99) and all‐cause mortality (HR 0.82, 95% CI 0.69–0.98) reduction. Detailed information on clinical outcome evaluation is presented in Figure 2 .
Figure 2.
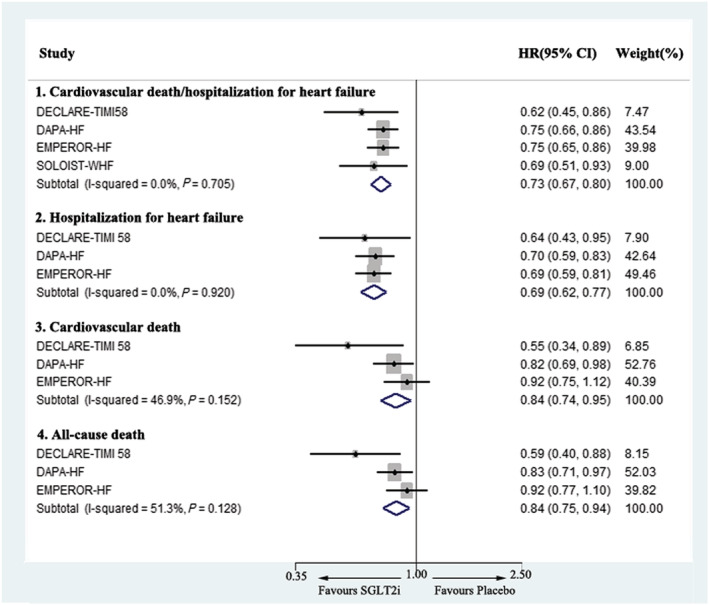
Meta‐analysis of the effects of SGLT2i on the composite of cardiovascular death or hospitalization for heart failure, cardiovascular death, hospitalization for heart failure, and all‐cause death in heart failure with reduced ejection fraction. CI, confidence interval; HR, hazard ratio; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
SGLT2i safety evaluation in patients with HFrEF
Drug safety is a matter of great concern during application. SGLT2i has been considered to be a possible cause of volume depletion, bone fracture, amputation, hypoglycaemia, urinary tract infection, or genital infection. The present meta‐analysis found that there were no significant differences between the SGLT2i and control groups in volume depletion (RR 1.11, 95% CI 0.96–1.27), fracture (RR 1.07, 95% CI 0.83–1.37), amputation (RR 1.31, 95% CI 0.81–2.13), major hypoglycaemia (RR 0.92, 95% CI 0.60–1.43), and urinary tract infection (RR 1.03, 95% CI 0.79–1.35). Only the risk of genital infection was significantly higher in the SGLT2i group (RR 2.78, 95% CI 1.46–5.29) (Figure 3 ).
Figure 3.
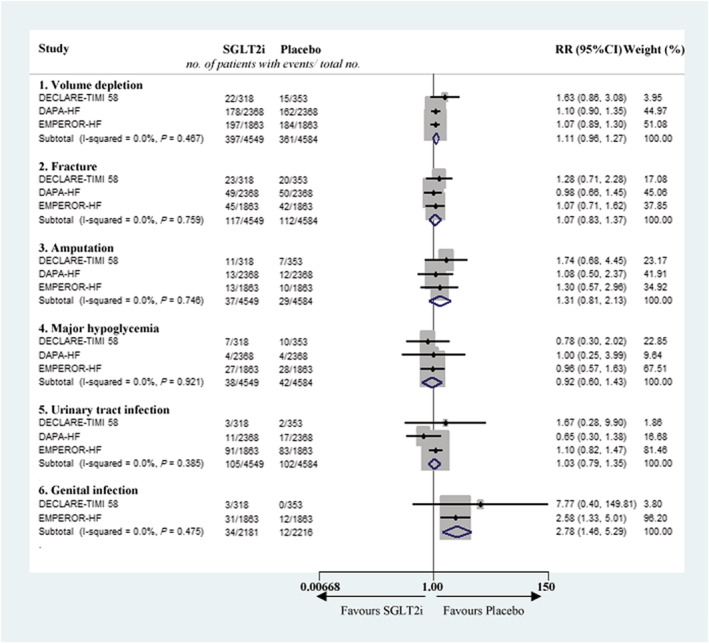
Meta‐analysis of safety evaluation of SGLT2i on volume depletion, fracture, amputation, major hypoglycaemia, urinary tract infection, and genital infection. CI, confidence interval; RR, risk ratio; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
SGLT2i in patients with or without DM
SGLT2i was first used as a drug for glucose control. It was unexpectedly found to have cardiovascular protective effects in patients with DM with a risk of cardiovascular disease. Previous research has also demonstrated a lower risk of cardiovascular death or hospitalization for heart failure in patients with HFrEF. However, whether the results in patients with HFrEF with or without DM would be the same needed additional exploration. The present pooled results showed that a reduction in cardiovascular death or hospitalization for heart failure was of similar magnitude in patients with HFrEF with or without DM, with a slightly lower HR in patients with DM (HR 0.71, 95% CI 0.64–0.80) when compared with patients without DM (HR 0.75, 95% CI 0.65–0.87) (Figure 4 ).
Figure 4.
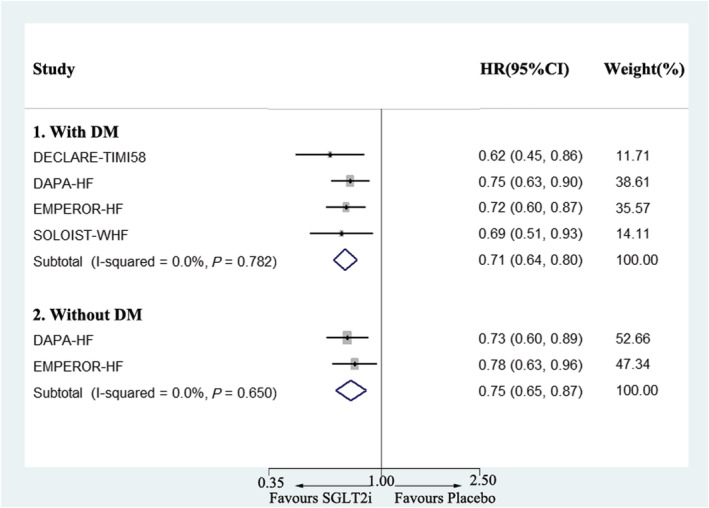
Meta‐analysis of the effects of SGLT2i on the composite of cardiovascular death or hospitalization for heart failure in heart failure with reduced ejection fraction with or without DM. CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
SGLT2i and ARNI comparison in patients with HFrEF
Both SGLT2i and ARNI have been shown to reduce the risk of cardiovascular events in patients with HFrEF. However, it remains unclear which one possesses a better prevention effect. This section was designed to explore this issue. Because there was no direct comparison evidence existed between these two therapy strategies, data analysis using the ITC method was performed with data from the included six trials (four for SGLT2i and two for ARNI). The comparator treatment for all included trials was recommended therapy for heart failure (containing ACEI/ARB, mineralocorticoid receptor antagonists, beta‐blockers, diuretics, digitalis, etc.). In order to ensure the accuracy of the result, patients in DAPA‐HF and EMPEROR‐HF using SGLT2i in combination with ARNI were excluded. Pooled results showed that SGLT2i and ARNI had similar effects on cardiovascular death or hospitalization for heart failure prevention in patients with HFrEF (HR 0.93, 95% CI 0.82–1.06) without significant heterogeneity (P = 0.28) (Figure 5 ).
Figure 5.
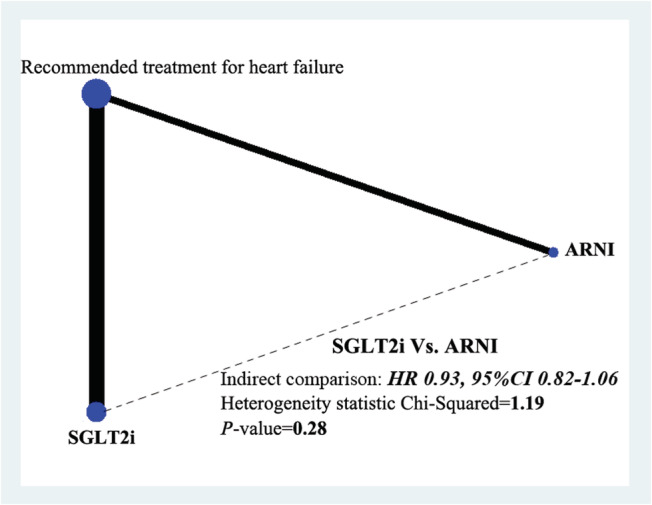
Network plot for the effects comparison between SGLT2i and ARNI on the composite of cardiovascular death or hospitalization for heart failure in heart failure with reduced ejection fraction with indirect comparison evidence from six included trials. CI, confidence interval; HR, hazard ratio; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
Combination application of SGLT2i and ARNI versus ARNI monotherapy
Because the aforementioned results showed similar prevention effects of SGLT2i and ARNI in patients with HFrEF, it was worth investigating whether combination usage of these two drugs would achieve better outcomes than a monotherapy. In the DAPA‐HF trial, 508 included patients (250 in the SGLT2i group and 258 in the control group) had taken ARNI instead of ACEI/ARB upon randomization. In the EMPEROR‐HF trial, 727 patients (340 vs. 387 in the treatment and control groups, respectively) were initially prescribed ARNI. The DAPA‐HF trial showed no significant difference in cardiovascular death or hospitalization for heart failure between SGLT2i + ARNI group and ARNI monotherapy group, while the EMPEROR‐HF trial demonstrated a significant reduction in the primary outcome in the SGLT2i + ARNI group. Pooled meta‐analysis data also indicated a lower occurrence of cardiovascular death or hospitalization for heart failure in patients with HFrEF taking both SGLT2i and ARNI (HR 0.68, 95% CI 0.53–0.89) (Figure 6 ).
Figure 6.
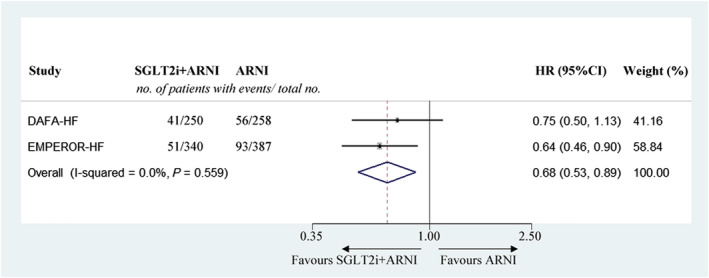
Meta‐analysis of comparing the effects of combined use of SGLT2i and ARNI versus monotherapy with ARNI on the composite of cardiovascular death or hospitalization for heart failure in heart failure with reduced ejection fraction. CI, confidence interval; HR, hazard ratio; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
Discussion
The present meta‐analysis identified excellent SGLT2i effects on cardiovascular death or hospitalization for heart failure prevention in patients with HFrEF, regardless of DM status. No significant differences were found in volume depletion, fracture, amputation, major hypoglycaemia, and urinary tract infection, except for genital infection. In addition, SGLT2i and ARNI had similar effects on reduction of cardiovascular death or hospitalization for heart failure risk. Combination use of SGLT2i and ARNI had a lower occurrence of cardiovascular death or hospitalization for heart failure compared with the ARNI‐only treatment.
SGLT2i has been found to be able to lower the level of both haemoglobin A1c and fasting blood glucose without relying on insulin secretion and sensitization. 17 It also plays a role in weight and visceral fat loss in patients with DM. 18 SGLT2i has been safely used in patients with DM without causing hypoglycaemia. EMPA‐REGOUTCOME was the first large‐scale RCT evaluating the effects of empagliflozin on cardiovascular morbidity and mortality in patients with type 2 diabetes and high cardiovascular risk. It demonstrated that empagliflozin prominently reduced the risk of death from cardiovascular causes and nonfatal myocardial infarction or nonfatal stroke by 14% (HR 0.86, 95% CI 0.74–0.99). 17 Then more studies were conducted to explore the effects of SGLT2i on cardiovascular protection. The CANVAS study demonstrated similar results, where patients treated with canagliflozin had a lower risk of cardiovascular events (HR 0.86, 95% CI 0.75–0.97). 19 However, the DECLARE‐TIMI 58 trial did not show a lower rate of a composite of major adverse cardiovascular events (HR 0.93, 95% CI 0.84–1.03) in dapagliflozin group. 19 All aforementioned three trials presented a significantly lower risk of cardiovascular death or hospitalization for heart failure and individual hospitalization. However, the effects on cardiovascular death remained controversial. Only the EMPA‐REG OUTCOME trial showed a significant reduction in cardiovascular death, while the CANVAS and DECLARE‐TIMI 58 trials demonstrated no significant differences in cardiovascular death prevention between the treatment and control groups. The three trials included 10–14% of patients with history of heart failure. After pooling these data together, the results demonstrated a lower rate of cardiovascular death or hospitalization for heart failure in the SGLT2i treatment group, indicating that patients with history of heart failure can also benefit from SGLT2i. 12
Subgroup analysis in the DECLARE‐TIMI 58 trial, in which patients were stratified on the basis of EF value, demonstrated a lower risk of cardiovascular events (including cardiovascular death, hospitalization for heart failure, and all‐cause death) in patients with HFrEF taking dapagliflozin. 20 In order to explore whether SGLT2i had similar effects on patients with HFrEF regardless of DM status, the DAPA‐HF and EMPEROR‐HF trials were conducted. 7 , 14 Their results showed that both dapagliflozin and empagliflozin had a lower rate of composite for cardiovascular death or hospitalization for heart failure and hospitalization for heart failure individually. However, there were differences in death risk (both cardiovascular death and all‐cause death) between the two trials. SGLT2i reduced the risk of cardiovascular death by 18% and all‐cause mortality by 17% with statistical significance in the DAPA‐HF trial compared with only 8% for each in the EMPEROR‐HF trial. The present meta‐analysis pooled results demonstrated a lower risk of both hospitalization for heart failure and death in the SGLT2i treatment group. However, moderate heterogeneity was present. The mean EF values in the DECLARE‐TIMI 58, DAPA‐HF, and EMPEROR‐HF trials were 38%, 31%, and 27%, respectively. It was difficult to determine whether patients with a higher EF value might benefit more from SGLT2i prescription in preventing cardiovascular and all‐cause mortality, because patients with a lower EF might have a poorer prognosis originally. Trials designed to confirm the different effects of SGLT2i on death prevention among patients with stratified EF values are in need.
Both patients with HFrEF with and without DM can benefit from SGLT2i in preventing cardiovascular death or hospitalization for heart failure, indicating that SGLT2i might improve HFrEF patient outcomes via pleiotropic effects, not just by lowering blood glucose levels. SGLT2i administration can lower tissue glucose disposal and stimulate lipolysis, lipid oxidation, and ketogenesis, and this fuel utilization shift might have a role in improving myocardial metabolism and cardiac function. 21 Animal experiments have shown that SGLT2i can reduce left ventricular mass, left ventricular end‐diastolic volume, and left ventricular end‐systolic volume. 22 A direct SGLT2i effect on human cardiac myofibroblast phenotype and function by attenuating myofibroblast activity and cell‐mediated collagen remodelling has been shown, 23 indicating that SGLT2i might improve ventricular constitution by attenuating fibrosis. In addition, SGLT2i has also been shown to have effects on blood pressure control, weight loss, and osmotic diuresis, which synergistically improve cardiac function. 11 , 24 , 25 Interestingly, SGLT2i has been approved to significantly reduce the cardiovascular events in patients with HFrEF, but it has no prevention effects on patients with heart failure without reduced EF. The second analysis of DECLARE‐TIMI 58 showed that SGLT2i reduced the incidence of cardiovascular death, hospitalization for heart failure, and all‐cause mortality in HFrEF (EF < 45%) with statistical significance but not in patients with EF ≥ 45%. 16 The same result was also noticed in the SOLOIST‐WHF trial; sotagliflozin group demonstrated lower risk of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure with statistical significance in patients with EF < 40%. But for patients with heart failure with EF of 40% to <50% or EF ≥ 50%, there is no significant difference found between the sotagliflozin group and the control group. 15
As another new pharmacological therapy for heart failure emerged in recent years, ARNI has been proven to be superior to ACEI in reducing the risk of cardiovascular events in patients with HFrEF. 6 , 13 Compared with enalapril, ARNI reduces the composite of death from cardiovascular causes or hospitalization for heart failure by 20% (HR 0.8, 95% CI 0.73–0.87) and death from any cause by 16% (HR 0.84, 95% CI 0.76–0.93) in PARADIGM‐HF. 6 These findings are also confirmed by real‐world studies. 26 There is a research showed that optimal implementation of ARNI therapy was empirically estimated to prevent nearly 28 484 deaths (range 18 230–41 017) each year. 27 The side effects that need to pay attention during application of ARNI are hypotension and non‐serious angioedema. 6 Due to the excellent effects on morbidity and mortality prevention, ARNI has been recommended (class I recommendation, moderate‐quality evidence) to patients with HFrEF by several guidelines for heart failure management. 28 , 29
Both SGLT2i and ARNI show excellent cardiovascular protective effects in patients with HFrEF, and it is quite intriguing and meaningful to determine which therapy is better. Our trial is the first meta‐analysis to evaluate this issue using existing indirect comparison data and ITC method. The result hints that SGLT2i and ARNI may serve as a substitute for each other in reducing cardiovascular death or hospitalization for heart failure in patients with HFrEF. But this conclusion may be more convincing with some direct comparison evidences. Because SGLT2i and ARNI take effect through different mechanisms, whether combined use of SGLT2i and ARNI could reinforce the cardiovascular protective effects with fewer side effects becomes a topic of great interest. Our study obtained the data of 1235 patients from DAPA‐HF and EMPEROR‐HF, and found that combination therapy was significantly better than ARNI monotherapy in patients with HFrEF. This finding provides us a new therapeutic regimen, which may help to obtain better prognosis in patients with HFrEF in the future.
Several limitations should be acknowledged in the present analysis. Firstly, aggregated study‐level data were used other than individual participant data. Secondly, the inclusion criteria for HFrEF patients in PIONEER‐HF trial was slightly different from others, and its follow‐up period was much shorter, which might result in some internal heterogeneity. Lastly, the exact definition of primary outcomes varied among the included trials, but only slightly.
Conclusions
SGLT2i can significantly and safely reduce the risk of cardiovascular death or hospitalization for heart failure in patients with HFrEF regardless of DM status. SGLT2i and ARNI have similar effects on cardiovascular death or hospitalization for heart failure prevention. And the combined use of SGLT2i and ARNI results in a better cardiovascular protective effect.
Conflict of interest
Yuling Yan, Bin Liu, Jun Du, Jing Wang, Xiaodong Jing, Yajie Liu, Songbai Deng, Jianlin Du, and Qiang She declare that they have no conflict of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770251), National Natural Science Foundation of China Youth Science Fund Project (81800254), Natural Science Foundation of Chongqing Science and Technology Commission (cstc2020jcyj‐ msxmX0210), and medical research projects of Chongqing Science and Technology Commission and Chongqing Health Committee (2021MSXM217, 2020FYYX047).
Supporting information
Figure S1. Supporting information.
Data S1. Supporting information.
Yan, Y. , Liu, B. , Du, J. , Wang, J. , Jing, X. , Liu, Y. , Deng, S. , Du, J. , and She, Q. (2021) SGLT2i versus ARNI in heart failure with reduced ejection fraction: a systematic review and meta‐analysis. ESC Heart Failure, 8: 2210–2219. 10.1002/ehf2.13313
References
- 1. Lee HY, Baek SH. Optimal use of beta‐blockers for congestive heart failure. Circ J 2016; 80: 565–571. [DOI] [PubMed] [Google Scholar]
- 2. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta‐analysis. BMC Cardiovasc Disord 2016; 16: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muneer K, Nair A. Angiotensin‐converting enzyme inhibitors and receptor blockers in heart failure and chronic kidney disease—demystifying controversies. Indian Heart J 2017; 69: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nochioka K, Sakata Y, Shimokawa H. Combination therapy of renin angiotensin system inhibitors and β‐blockers in patients with heart failure. Adv Exp Med Biol 2018; 1067: 17–30. [DOI] [PubMed] [Google Scholar]
- 5. Chia N, Fulcher J, Keech A. Beta‐blocker, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, nitrate‐hydralazine, diuretics, aldosterone antagonist, ivabradine, devices and digoxin (BANDAID2): an evidence‐based mnemonic for the treatment of systolic heart failure. Intern Med J 2016; 46: 653–662. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 8. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 9. Burnett H, Earley A, Voors AA, Senni M, McMurray JJ, Deschaseaux C, Cope S. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Circ Heart Fail 2017; 10: e003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose‐lowering effect. Cardiovasc Diabetol 2020; 19: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sternlicht H, Bakris GL. Blood pressure lowering and sodium‐glucose co‐transporter 2 inhibitors (SGLT2is): more than osmotic diuresis. Curr Hypertens Rep 2019; 21: 12. [DOI] [PubMed] [Google Scholar]
- 12. Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause‐Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. N Engl J Med 2019; 139: 2516–2527. [DOI] [PubMed] [Google Scholar]
- 13. Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, Gurmu Y, McCague K, Rocha R, Braunwald E. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER‐HF trial. Circulation 2019; 139: 2285–2288. [DOI] [PubMed] [Google Scholar]
- 14. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt DL, Szarek M, Steg PG. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 16. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 18. Singh AK, Unnikrishnan AG, Zargar AH, Kumar A, Das AK, Saboo B, Sinha B, Gangopadhyay KK, Talwalkar PG, Ghosal S, Kalra S, Joshi S, Sharma SK, Sriram U, Mohan V. Evidence‐based consensus on positioning of SGLT2i in type 2 diabetes mellitus in Indians. Diabetes Ther 2019; 10: 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 20. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL. Dapagliflozin and cardiovascular outcomes in type 2. Diabetes 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 21. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium‐glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016; 65: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 22. Santos‐Gallego CG, Requena‐Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia‐Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol 2019; 73: 1931–1944. [DOI] [PubMed] [Google Scholar]
- 23. Kang S, Verma S, Hassanabad AF, Teng G, Belke DD, Dundas JA, Guzzardi DG, Svystonyuk DA, Pattar SS, Park DSJ, Turnbull JD, Duff HJ, Tibbles LA, Cunnington RH, Dyck JRB, Fedak PWM. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA‐REG OUTCOME results. Can J Cardiol 2020; 36: 543–553. [DOI] [PubMed] [Google Scholar]
- 24. Kario K, Ferdinand KC, O'Keefe JH. Control of 24‐hour blood pressure with SGLT2 inhibitors to prevent cardiovascular disease. Prog Cardiovasc Dis 2020; 63: 249–262. [DOI] [PubMed] [Google Scholar]
- 25. Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Vecchis R, Ariano C, Di Biase G, Noutsias M. Sacubitril/valsartan for heart failure with reduced left ventricular ejection fraction: a retrospective cohort study. Herz 2019; 44: 425–432. [DOI] [PubMed] [Google Scholar]
- 27. Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol 2016; 1: 714–717. [DOI] [PubMed] [Google Scholar]
- 28. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 29. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting information.
Data S1. Supporting information.


