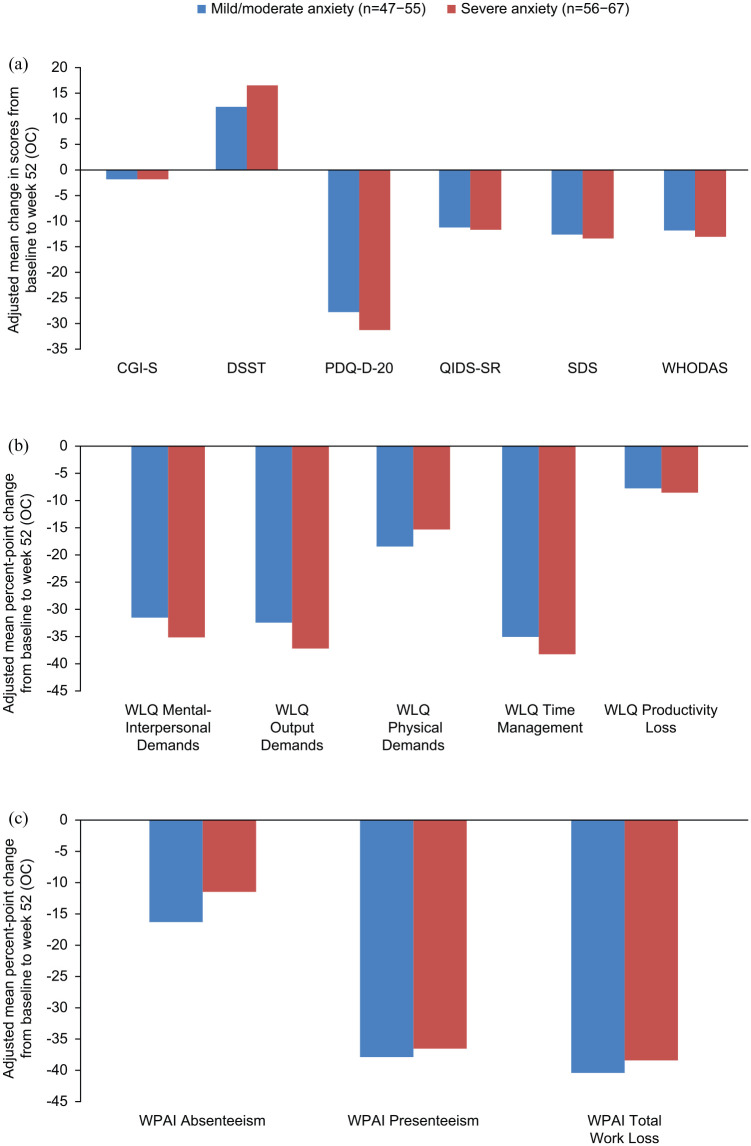
Figure 3.
Baseline-adjusted improvements in (a) clinical and functioning outcomes, (b) WLQ outcomes, and (c) WPAI outcomes after 52 weeks of vortioxetine treatment according to severity of anxiety symptoms at baseline (full analysis set). For all scales except the DSST, higher scores indicate more severe impairment; for the DSST, higher scores indicate better cognitive performance.
CGI-S, Clinical Global Impressions–Severity scale (score range 1–7); DSST, Digit Symbol Substitution Test (score range 0–100); GAD-7, Generalized Anxiety Disorder 7-item (total score range 0–21); OC, observed cases; PDQ-D-20, 20-item Perceived Deficits Questionnaire-Depression (total score range 0–80); QIDS-SR, Quick Inventory of Depression Symptomatology–Self Report (total score range 0–27); SDS, Sheehan Disability Scale (total score range 0–30); WHODAS, World Health Organization Disability Assessment Schedule 2.0 (total score range 0–100); WLQ, Work Limitations Questionnaire (total score range 0–25%); WPAI, Work Productivity and Activity Impairment (total score range 0–100%).