Fig. 5 |. Resolution and speed of custom and commercial light-sheet microscopes.
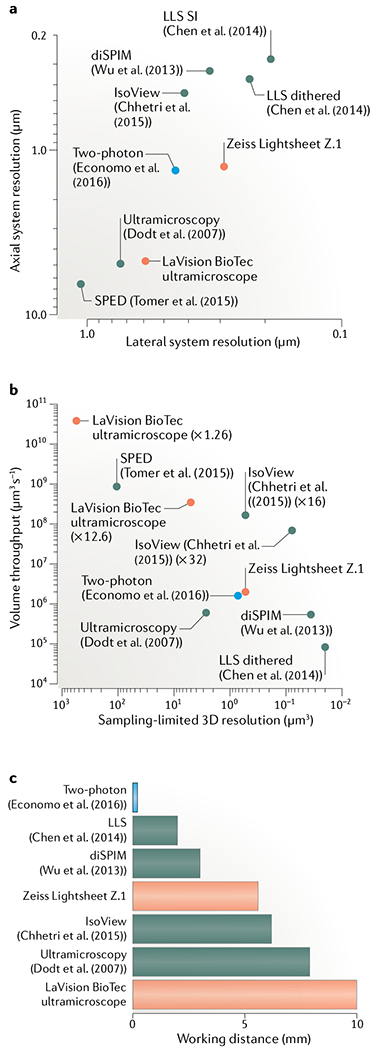
a | Lateral versus axial system resolution of custom14,105,110–112 (red) and commercial166,167 (green) light-sheet microscopes and state-of-the-art two-photon microscopes54 (blue). System resolution values168 representthe optical configurations and light-sheet properties reported in each study for the demonstration experiments shown in part b. The guantification disregards spatial sampling limitations inherent to the choice of detector. Resolution values are provided as the full width at half maximum (FWHM) size of the system point spread function. Lattice light-sheet (LLS) microscopy uses two different acquisition modes (dithered light sheet versus structured illumination (SI)), which affect the speed and resolution. b | Sampling-limited 3D resolution versus volume throughput for commercial light-sheet microscopes (green) and imaging experiments performed with custom light-sheet (black) and two-photon (blue) microscopes. 3D resolution is defined as , where dlat is the sampling-limited lateral FWHM size and dlat is the sampling-limited axial FWHM size of the point spread function. Lateral sampling can be adjusted by changing the magnification of the detection system, which typically affects both volume throughput and effective resolution (for examples, see ×1.26 versus ×12.6 magnification settings for the LaVision BioTec ultramicroscope or ×16 versus ×32 magnification settings for IsoView). Data points for custom light-sheet and two-photon microscopy refer to imaging of hippocampal dendrites14, Caenorhabditis elegans development111, cellular dynamics110, zebrafish nervous system105–112 and mouse brains54. Data points for commercial microscopes reflect the technical specifications provided by the manufacturer165,166. All techniques included in the plot have been successfully applied to the imaging of cleared tissues. c | Working distance of the detection systems for the methods shown in parts a and b. The design by Economo et al.54 does not require long-working-distance optics for imaging mouse brains since their microscope is integrated with a tissue vibratome. Chhetri et al.112 surrounded the specimen with four identical objectives for illumination and fluorescence detection, which allows an increase in the bidirectional working distance by a factor of 2 (compared with the native working distance of a single objective) in the special case of imaging transparent, cleared tissues. The maximum supported specimen size can in principle also be doubled in other light-sheet techniques at the expense of temporal resolution by rotating the specimen by 180° and acquiring volumetric data from this opposite view. Tomer et al.105 proposed using the majority of the detection objective’s working distance to introduce a block of high-refractive-index material in the detection path. The effective working distance (distance between the block and the in-focus region of the specimen) is thus typically not identical to the native working distance of the objective. For this reason, the latter technique is not included in this plot.
