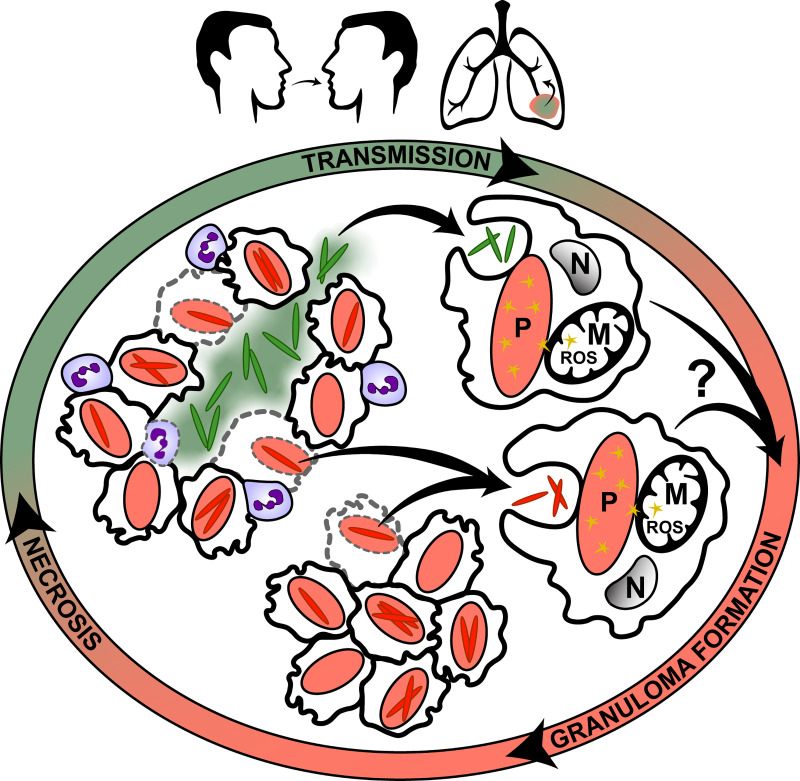
Fig 6. Changes in [Zn2+] throughout TB infection cycle drives formation of Zn2+-limited Mtb with anticipatory adaptations.
The gradient around the outside of the figure represents the cycle of changing [Zn2+] throughout infection; red indicates Zn2+-replete and green indicates Zn2+-limited microenvironments. These [Zn2+]-defined microenvironments drive formation of physiologically distinct subpopulations of Mtb with Zn2+-replete Mtb (red rods) in phagosomes and Zn2+-limited Mtb (green rods) exposed to CP, e.g., in the caseum. After phagocytosis of Mtb, solid granulomas form (bottom) and sustained inflammation leads to the recruitment of neutrophils (purple) which cause necrosis (left). Mtb may be transmitted from necrotic or apoptotic cells (grey dashed margins) in either solid or necrotic granulomas. With the latter, Mtb transit through the Zn2+-limited caseum before being transmitted host-to-host or within a single infected individual (top). The Zn2+-limited Mtb subpopulation has adaptations that could enable this subpopulation to anticipate forthcoming stress and resist host killing (e.g., oxidative stress–yellow stars) and/or affect immune cell activation. The exact mechanisms of how [Zn2+]-derived changes in physiology of Mtb may affect disease outcome are unclear and we highlight the importance of defining the host response to Zn2+-replete and Zn2+-limited Mtb (question mark). Drawings are representative and not to scale and some components of granulomas have been omitted for clarity. Abbreviations: nucleus (N), mitochondria (M), phagosome (P).