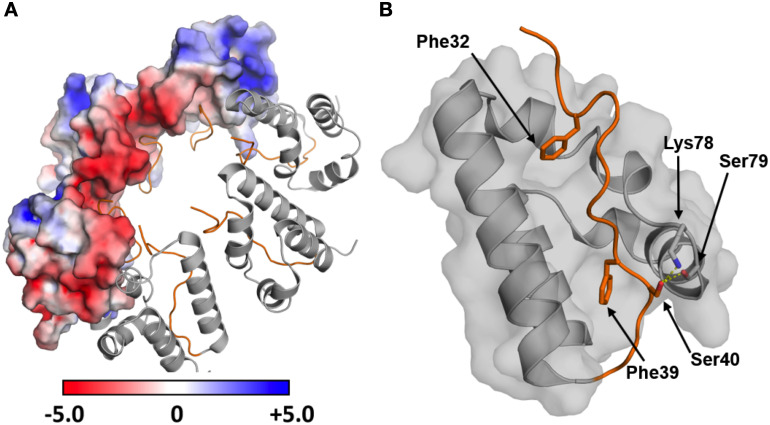
Fig 3. The N-terminal region preceding dArc-NL packs into the hydrophobic core of the domain and leads to the formation of the capsid hexamer.
(A) The hexameric form of dArc2-NL observed in the capsid (PDB: 6TAQ; [21]), showing the electrostatic surface potential for half of the monomers. The canonical fold enables contact formation between the oppositely charged surfaces of each monomer. The N-terminal tail is showed in orange. (B) Residues contributing to the packing of the N-terminal tail (orange) into the capsid hexamer. Phe32 and Phe39 pack into two exposed pockets in the hydrophobic core. Further interactions are observed for Ser40, which hydrogen bonds directly with Lys78 and Ser79 in the α2 kink (yellow dashed lines).