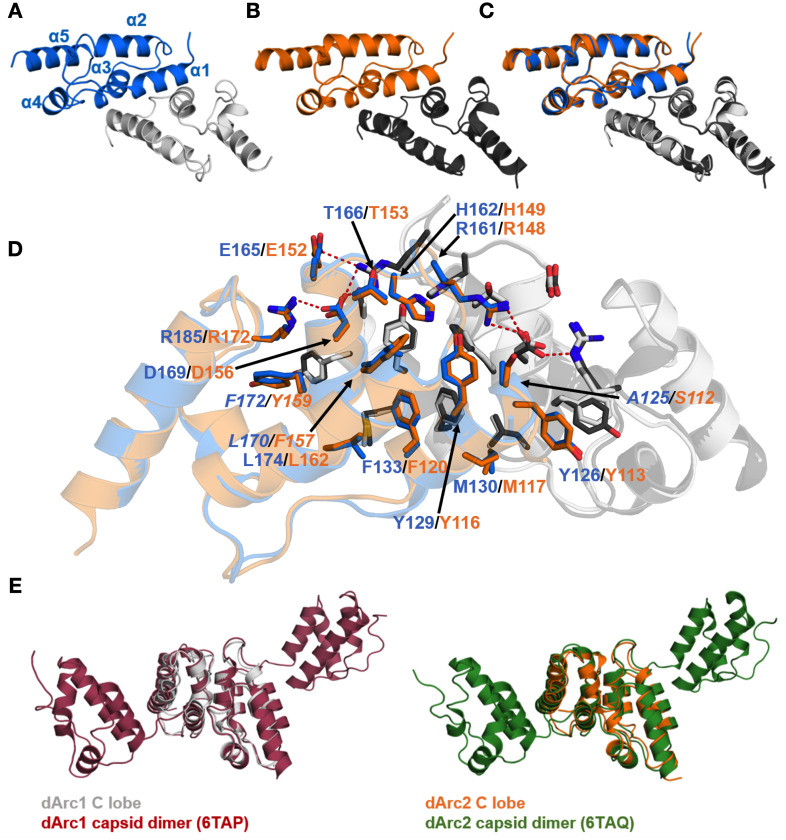
Fig 6. Crystal structures of the dArc1 and dArc2 C-lobes.
(A) dArc1-CL. (B) dArc2-CL. (C) The two structures, which deviate with an all-atom RMSD of 0.48 Å, superimposed. (D) Residues contributing to the dimer interface in dArc-CL. dArc1-CL residues are marked in blue, and residues of dArc2-CL are marked in orange. Variable residues are indicated in italics. All residues contributing to the dimer interface are conserved, with the exception of A125 (dArc1) which corresponds to S112 (dArc2). Polar interactions are shown with red dashed lines. (D) A comparison of the dArc-CL crystal structures with the same domains in dArc capsids. Both the dArc1 and dArc2 C-lobes closely resemble their counterparts in the capsids, with an all-atom RMSD of 1.75 Å2 and 1.13 Å2, respectively.