Fig. 2. The structure of the prM-E heterodimer reveals a furin site buried within the spike and a central pillar supporting the spike rather than the proposed drawstring.
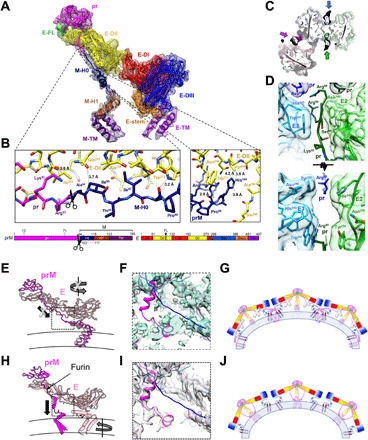
(A) The electron density and structure of the fivefold prM-E heterodimer. The color scheme matches the domain diagram (bottom), which also indicates glycans (cyan), furin cleavage site (scissors), and unmodeled region (dashed line). (B) Zoom of the prM linker. (C) Section of the trimeric spike near the furin site viewed from virion exterior. Molecular surface and stick representation are colored according to the prM-E proximity to the twofold (green/lime), threefold (blue/cyan), and fivefold (magenta/pink) symmetry axes. (D) Zooms of the prM/E interfaces as in (C) (bottom) or in orthogonal orientation (top). (E and F) Structure of BinJV (E) compared to a main-chain model of immature DENV-1 [(H), PDB: 4B03]. E and prM is in pink and magenta, respectively. Arrows indicate that movements proposed in the drawstring model (E) are incompatible with the BinJV topology, supporting a prM-guided collapse (H). (F and I) Linker region electron density for BinJV (F) and DENV-1 [(I) EMD-2141]. Density is only visible for the BinJV model (magenta). None of the reconstructions show density for the proposed DENV-1 prM linker (blue; PDB: 4B03). (G and J) Schematics of the immature virion colored as (A) with membrane in blue. Instead of the previously proposed domain swap (G), prM forms a supporting pillar at the center of each BinJV spike (J).
