Fig. 2. CUL3-KBTBD7 acts as an E3 ubiquitin ligase for Vangl.
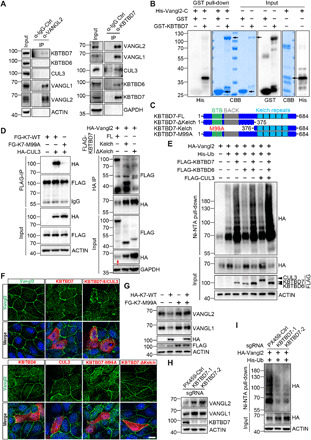
(A) Endogenous interaction between Vangl and KBTBD7 in HEK293T cells. Vangl2 binds to KBTBD7 and Vangl1 but not KBTBD6 or CUL3 (left). KBTBD7 binds to Vangl1, Vangl2, CUL3, and KBTBD6 (right). IgG, immunoglobulin G. (B) KBTBD7 directly binds to Vangl2. Glutathione S-transferase (GST)–fused KBTBD7 and His-fused C-terminal Vangl2 (amino acids 254 to 521) recombinant proteins were coincubated and subjected to GST pull-down. CBB, Coomassie brilliant blue staining. (C) Schematic domain structures of various KBTBD7. M99A, methionine to alanine mutation within the BTB domain. (D) Interaction between various Flag-tagged KBTBD7 (FG-K7) and HA-tagged CUL3 or Vangl2 in HEK293T cells. KBTBD7-M99A mutation abolished its binding to CUL3 (left). Kelch repeats of KBTBD7 were required for binding to Vangl2 (right). Note that overexpressed HA-Vangl2 was largely degraded by full-length KBTBD7 (red arrow in input). (E) KBTBD7 alone or with KBTBD6 and CUL3, but not KBTBD6 or CUL3 alone, promoted Vangl2 ubiquitination in HEK293T cells. (F) KBTBD7 abolished the plasma membrane localization of Vangl2. KBTBD6, CUL3, or mutant KBTBD7 was incapable of degrading Vangl2. Endogenous Vangl2 (green) in Madin-Darby canine kidney (MDCK) cells was examined by immunofluorescent staining (IF). The cells expressing KBTBD6, KBTBD7, or CUL3 were marked by FLAG IF (red). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 10 μm. (G) KBTBD7-M99A mutant was unable to degrade Vangl proteins but showed a dominant negative effect on wild-type (WT) KBTBD7 (K7). (H) Loss of KBTBD7 in HEK293T cells increased endogenous Vangl1 and Vangl2 protein levels. KBTBD7 was knocked out in HEK293T cells by CRISPR-Cas9–mediated genome editing, and two single KBTBD7 null clones were analyzed. The PX459 empty vector served as a control. (I) Loss of KBTBD7 decreased Vangl2 ubiquitination.
