Fig. 8. Model of ERAD-mediated regulation of Vangl.
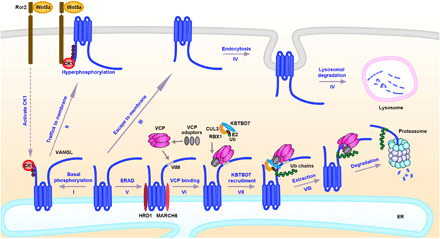
(I) Newly synthesized Vangl proteins are basally phosphorylated by CK1 in the ER; (II) the basal phosphorylated Vangl are transported to the cell surface where they undergo further phosphorylation and become stabilized; (III) some of unmodified Vangl escape ERAD and reach the cell surface; (IV) the unphosphorylated Vangl are not stable and are internalized and degraded via the lysosomal pathway; (V) most of the unmodified or unfolded Vangl proteins are degraded through the ERAD pathway. ERAD components HRD1 and MARCH6 are required for the degradation of Vangl and may initiate Vangl ubiquitination. (VI) VCP directly binds to Vangl at a highly conserved VIM; (VII) VCP recruits cytosolic E3 ligase KBTBD7 via its UBA-UBX adaptors (SAKS1, UBXD7, and FAF1), resulting in enhanced poly-ubiquitination of Vangl; and (VIII) extraction of highly ubiquitinated Vangl molecules for proteasomal degradation.
