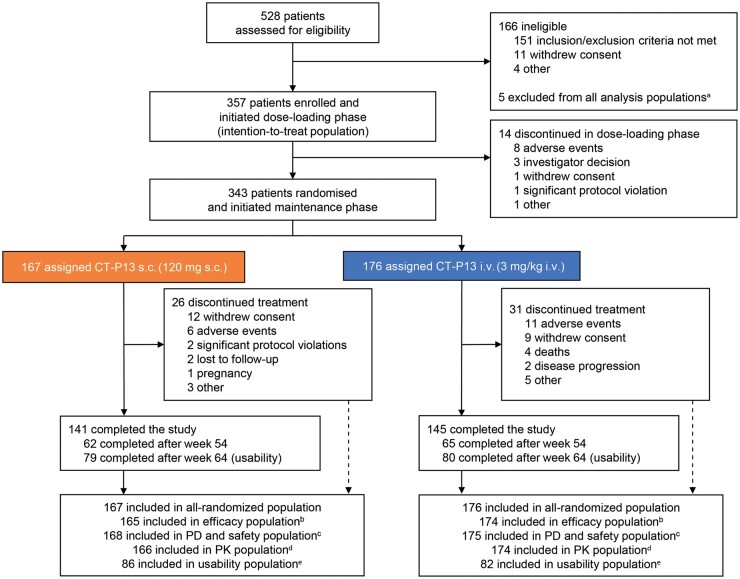
Fig. 1.
Patient disposition
aFive patients were excluded from all analysis populations because of significant Good Clinical Practice non-compliance of the study centre.
bThree patients (1 in the CT-P13 s.c. arm and 2 in the i.v. arm) with at least one major protocol deviation and 1 patient (in the CT-P13 s.c. arm) with no efficacy assessment after week 6 were excluded from the efficacy population.
cOne patient randomized to the CT-P13 i.v. arm received CT-P13 s.c. treatment instead of placebo s.c. treatment at week 14, thus receiving both CT-P13 i.v. 3 mg/kg and CT-P13 s.c. 120 mg at week 14. This patient was included in the CT-P13 s.c. arm for the PD and safety populations and excluded from the efficacy and PK populations.
dOne patient with a major protocol deviation (CT-P13 i.v. arm) and 2 patients without PK concentration data (1 in each of the CT-P13 s.c. and i.v. arms) were excluded from the PK population.
eAll 168 patients eligible for usability assessment (patients in Bulgaria, Poland and Russian Federation continuing the study at week 46) were included in the usability population.
PD, pharmacodynamics; PK, pharmacokinetics.