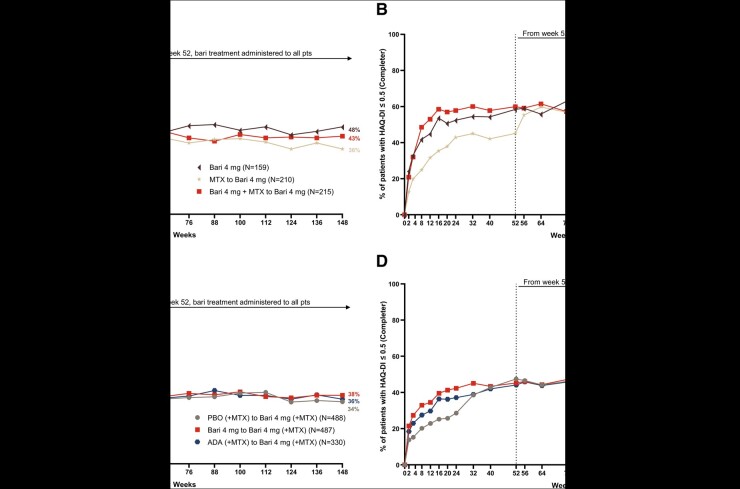
Fig. 3.
Patients who achieved HAQ-Disability Index (HAQ-DI) ≤0.5 in RA-BEGIN and RA-BEAM trials
(A) and (B) illustrate the percentage of patients who achieved HAQ-DI ≤ 0.5 over time based on the NRI and completer analyses in RA-BEGIN and RA-BEYOND. (C) and (D) illustrate the percentage of patients who achieved HAQ-DI ≤ 0.5 over time based on the NRI and completer analyses in RA-BEAM and RA-BEYOND. †In RA-BEGIN, rescue was offered at week 24.
‡In RA-BEAM, rescue was offered at week 16. At week 24, all PBO (+MTX) patients were switched to baricitinib 4 mg (+MTX).
§Upon entering RA-BEYOND at week 52, patients who received MTX and Bari + MTX in RA-BEGIN were switched to Bari 4 mg monotherapy; patients who received ADA + (MTX) in RA-BEAM were switched to Bari 4 mg (+MTX). Data points are listed in Supplementary Tables S5 and S6, available at Rheumatology online.
ADA, adalimumab; Bari, baricitinib; HAQ-DI, HAQ-Disability Index; NRI, non-responder imputation; PBO, placebo; pts, patients.